Capivasertib plus fulvestrant in patients with HR-positive/HER2-negative advanced breast cancer: phase 3 CAPItello-291 study extended Chinese cohort
- PMID: 40346047
- PMCID: PMC12064754
- DOI: 10.1038/s41467-025-59210-6
Capivasertib plus fulvestrant in patients with HR-positive/HER2-negative advanced breast cancer: phase 3 CAPItello-291 study extended Chinese cohort
Abstract
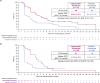
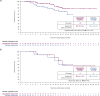
In the global CAPItello-291 randomized phase 3 study (NCT04305496) in patients with hormone receptor-positive/HER2-negative advanced breast cancer and progression during/after aromatase inhibitor treatment, capivasertib-fulvestrant significantly improved progression-free survival (PFS) in the overall population and patients with PIK3CA/AKT1/PTEN-altered tumors versus placebo-fulvestrant. We assessed efficacy and safety of capivasertib-fulvestrant in a prespecified exploratory analysis of a Chinese cohort (n = 24) and extended study with the same protocol (n = 110). Clinically meaningful PFS benefit for capivasertib-fulvestrant was observed in the overall population (median PFS: 6.9 [capivasertib-fulvestrant] versus 2.8 [placebo-fulvestrant] months; hazard ratio 0.51, 95% CI 0.34-0.76), patients with PIK3CA/AKT1/PTEN-altered tumors (n = 46; 5.7 versus 1.9 months; hazard ratio 0.41, 95% CI 0.19-0.85) and PIK3CA/AKT1/PTEN-non-altered tumors (patients with confirmed next-generation sequencing results [n = 68]; 9.2 versus 2.7 months; hazard ratio 0.38; 95% CI 0.21-0.68). The most frequent adverse events (AEs) with capivasertib-fulvestrant were diarrhea (60.6% versus 11.3% with placebo-fulvestrant) and hyperglycemia (57.7% versus 17.7%). AEs leading to capivasertib-fulvestrant discontinuation were reported in 11.3% of patients versus 3.2% for placebo-fulvestrant. The benefit-risk profile of capivasertib-fulvestrant in the Chinese cohort was favorable; further exploration in patients with PIK3CA/AKT1/PTEN-non-altered tumors is warranted.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: X.H. has participated on advisory boards for AstraZeneca, received institutional funding for an investigator-initiated trial from Merck Sharpe and Dohme, and has served as a local Principal Investigator for AstraZeneca and Novartis. Y-S.L. has participated on advisory boards for Novartis, Eli Lilly, Merck Sharpe and Dohme, Roche, Pfizer, AstraZeneca, Eisai, Daiichi Sankyo, and Gilead Sciences, has been an invited speaker for Novartis, Eli Lilly, Merck Sharpe and Dohme, Roche, Pfizer, AstraZeneca, Eisai, Daiichi Sankyo, and Gilead Sciences, has served as a local Principal Investigator for Novartis, Roche, Pfizer, Merck Sharpe and Dohme, Eli Lilly, Eisai, AstraZeneca, Gilead Sciences and Jellox, has served as a chair/co-chair of a clinical trial steering committee for Novartis, and has held an uncompensated advisory role for AstraZeneca. J.M.C., E.F., L.J., and X.Z. are full-time employees of AstraZeneca. N.C.T. has participated in advisory boards AstraZeneca, Eli Lilly, Exact Sciences, Gilead Sciences, GlaxoSmithKline, Guardant Health, Inivata, Novartis, Pfizer, Roche/Genentech, Relay Therapeutics, Repare Therapeutics and Zentalis Pharmaceuticals, has received institutional research funding from AstraZeneca, Inivata, Invitae, Merck Sharpe and Dohme, Natera, Personalis, Pfizer and Roche/Genentech, and has received research assays/materials from Guardant Health and Bio-Rad. Q.Z., T.S., H.X., W.L., Y.T., L-M.T., M.Y., H.L., D.P., S-C.C., W.C., O.J., J.W., X. Wu, Xian Wang, A.Z. and Xiaojia Wang declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

