Iron-catalyzed sequential hydrosilylation
- PMID: 40346082
- PMCID: PMC12064691
- DOI: 10.1038/s41467-025-59364-3
Iron-catalyzed sequential hydrosilylation
Abstract
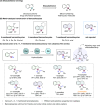
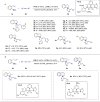
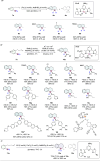
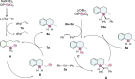
Highly regio-, diastereo- and enantioselective iron-catalyzed sequential hydrosilylation of o-alk-n-enyl-phenyl silanes with alkynes is reported for various 5-, 6-, and 7-membered benzosilacycles in 60-94% yields with up to 95:5 rr, 95:5 dr, and 99% ee. Chiral fully carbon-substituted silicon-stereogenic benzosilacycles could also be obtained via triple hydrosilylation reactions. The unique electronic effect of ligands is observed while adjusting the regioselectivity and enantioselectivity in hydrosilylation reactions. A possible mechanism has been proposed by variable time normalization analysis (VTNA) and H/D exchange experiment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures




Similar articles
-
Cobalt-Catalyzed Regio-, Diastereo- and Enantioselective Intermolecular Hydrosilylation of 1,3-Dienes with Prochiral Silanes.Angew Chem Int Ed Engl. 2022 Jul 25;61(30):e202205624. doi: 10.1002/anie.202205624. Epub 2022 Jun 9. Angew Chem Int Ed Engl. 2022. PMID: 35606326
-
Asymmetric Synthesis of Silicon-Stereogenic Vinylhydrosilanes by Cobalt-Catalyzed Regio- and Enantioselective Alkyne Hydrosilylation with Dihydrosilanes.Angew Chem Int Ed Engl. 2018 May 22;57(21):6319-6323. doi: 10.1002/anie.201802806. Epub 2018 Apr 25. Angew Chem Int Ed Engl. 2018. PMID: 29624830
-
Regio- and Enantioselective Cobalt-Catalyzed Sequential Hydrosilylation/Hydrogenation of Terminal Alkynes.Angew Chem Int Ed Engl. 2017 Jan 9;56(2):615-618. doi: 10.1002/anie.201610121. Epub 2016 Nov 30. Angew Chem Int Ed Engl. 2017. PMID: 27901300
-
Regio-, Diastereo-, and Enantioselective Hydrosilylation of Alkyl gem-Difluoroalkenes to Construct Carbon and Silicon Stereogenic Centers.Angew Chem Int Ed Engl. 2025 Aug 25;64(35):e202506157. doi: 10.1002/anie.202506157. Epub 2025 Jul 31. Angew Chem Int Ed Engl. 2025. PMID: 40607960
-
Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, Non-Thermally Triggered Hydrosilylation Reactions, and Industrial Applications.Polymers (Basel). 2017 Oct 20;9(10):534. doi: 10.3390/polym9100534. Polymers (Basel). 2017. PMID: 30965835 Free PMC article. Review.
References
-
- Wang, D. & Chan, T.-H. Chiral organosilicon compounds in asymmetric synthesis. Chem. Rev.92, 995–1006 (1992).
-
- Li, L., Lai, G.-Q., Jiang, J.-X. & Xu, L.-W. The recent synthesis and application of silicon-stereogenic silanes: A renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev.40, 1777–1790 (2011). - PubMed
-
- Zhao, W.-T., Gao, F. & Zhao, D.-B. Recent progress in σ-bond cross-exchange reactions to access diverse silacycles. Synlett29, 2595–2600 (2018).
-
- Ye, F. & Xu, L.-W. A glimpse and perspective of current organosilicon chemistry from the view of hydrosilylation and synthesis of silicon-stereogenic silanes. Synlett32, 1281–1288 (2021).
Grants and funding
LinkOut - more resources
Full Text Sources

