Anti-HBs persistence and anamnestic response among medical interns vaccinated in infancy
- PMID: 40346085
- PMCID: PMC12064783
- DOI: 10.1038/s41598-025-00055-w
Anti-HBs persistence and anamnestic response among medical interns vaccinated in infancy
Abstract
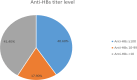
Medical interns are at high risk of acquiring Hepatitis B Virus (HBV) infection during their training. HBV vaccination is the most effective measure to reduce the global incidence of HBV. The duration of protection after HBV vaccination is still controversial. We aimed to determine the prevalence of protective anti-HBs levels among medical interns who had received compulsory hepatitis B vaccination in infancy, and to assess the anamnestic response of those subjects with non-protective antibodies titers, to a booster dose of the vaccine. We conducted a cross-sectional study on 519 medical interns in 2022. We examined their immunization status and records. Blood samples were collected and qualitative testing of Hepatitis B surface antigen (HBsAg), and quantitative testing of Hepatitis B surface antibody (anti-HBs) were performed. For medical interns whose titers were ˂ 10 mIU/mL, a booster dose of the vaccine was given, followed by repeat testing of anti-HBs 2 months later. About 304 (58.6%) of the medical interns revealed titers higher than or equal 10 mIU/mL. About (44.93%) of male medical interns showed an immunity level below 10 mIU/mL. However, (43.91%) of female medical interns had an antibody titer of 100 mIU/mL or higher. All subjects who got a booster dose presented a titer level of 10 mIU/mL 2 months later. About (41.4%) of our medical interns enrolled in the study had anti-HBs titer ˂ 10 mIU/mL. This raises the importance of establishing a screening protocol and offering booster dose for those at risk to protect them against the high risk of infection.
Keywords: Booster; Egypt; Hepatitis B; Immunization; Vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and informed consent for publication: The study was approved by the Research Ethics Committee of Alexandria University Medical School (Federal Wide serial number: 0306425). The research concept and aim were explained to all participating medical interns, and informed consent was signed before participating in the study. All the methods were performed in accordance with relevant guidelines and regulations. Recommendations: More date regarding specialty and history of sharp injuries would be considered in further researches that may be guided by this work.
Figures
References
-
- World Health Organization (WHO). Occupational Health, Health Workers and Health Worker Occupational Health. Available online: http://www.who.int/occupational_health/topics/hcworkers/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical