Impact of COVID-19 vaccination on symptoms and immune phenotypes in vaccine-naïve individuals with Long COVID
- PMID: 40346201
- PMCID: PMC12064684
- DOI: 10.1038/s43856-025-00829-3
Impact of COVID-19 vaccination on symptoms and immune phenotypes in vaccine-naïve individuals with Long COVID
Abstract
Background: The symptomatic and immune responses to COVID-19 vaccination of people with Long COVID are poorly characterized.
Methods: In this prospective study, we evaluated changes in symptoms and immune responses after COVID-19 vaccination in 16 vaccine-naïve individuals with Long COVID. Surveys were administered before vaccination and at 2, 6, and 12 weeks after receiving the first vaccine dose of the primary series. Simultaneously, SARS-CoV-2-reactive TCR enrichment, SARS-CoV-2-specific antibody responses, antibody responses to other viral and self-antigens, and circulating cytokines were quantified before vaccination and at 6 and 12 weeks after vaccination.
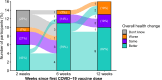
Results: At 12 weeks post-vaccination, self-reported improved health is seen in 10 out of 16 participants, 3 have no change, and 3 have worse health although 2 report transient improvement after vaccination. One participant reporting worse health was hospitalized twice with chest pain (after each dose). Symptom outcomes are most associated with plasma biosignatures. Higher baseline sIL-6R is associated with symptom improvement, and stably elevated levels of IFN-β and CNTF are associated with no improvement. Significant elevation in SARS-CoV-2-specific TCRs and spike protein-specific IgG are observed at 6 and 12 weeks after vaccination. No changes in reactivities are observed against herpes viruses and self-antigens.
Conclusions: In this study of 16 people with Long COVID, vaccination is associated with increased SARS-CoV-2 spike protein-specific IgG and T cell expansion in most participants. Specific immune features are associated with symptom change after vaccination and most participants experience improved health or no change following vaccination.
Plain language summary
The impact of the COVID-19 vaccine on unvaccinated individuals suffering from Long COVID is uncertain. This study assessed the experience and biological markers of 16 unvaccinated participants with Long COVID. A total of 10 participants had improved health after vaccination, three reported worsening health, with one hospitalized twice with chest pain. Vaccination boosted the body’s immune responses against the virus that causes COVID-19. We identified biological markers that correlate with the changes in overall health after vaccination. Given that the study was small, more research is needed to confirm these results.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: In the past three years, H.M.K. received expenses and/or personal fees from United Health, Element Science, Eyedentifeye and F-Prime; he is a co-founder of Refactor Health and HugoHealth; and is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from the Food and Drug Administration, Johnson & Johnson, Google and Pfizer. A.I. co-founded and consults for RIGImmune, Xanadu Bio and PanV and is a member of the Board of Directors of Roche Holding and Genentech. No other authors declare competing interests.
Figures



Update of
-
Impact of COVID-19 vaccination on symptoms and immune phenotypes in vaccine-naïve individuals with Long COVID.medRxiv [Preprint]. 2024 Jan 12:2024.01.11.24300929. doi: 10.1101/2024.01.11.24300929. medRxiv. 2024. Update in: Commun Med (Lond). 2025 May 9;5(1):163. doi: 10.1038/s43856-025-00829-3. PMID: 38260484 Free PMC article. Updated. Preprint.
References
-
- Iacobucci, G. Long covid: Damage to multiple organs presents in young, low risk patients. BMJ m4470, 10.1136/bmj.m4470 (2020).
-
- Greenhalgh, T., Knight, M., A’Court, C., Buxton, M. & Husain, L. Management of post-acute covid-19 in primary care. BMJ370, m3026 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

