Oral Supplementation of n-3 Polyunsaturated Fatty Acids (n-3-PUFA) Can Prevent TBI-Induced Visual, Motor, and Emotional Deficits in Mice
- PMID: 40346443
- PMCID: PMC12367975
- DOI: 10.1007/s12035-025-05019-9
Oral Supplementation of n-3 Polyunsaturated Fatty Acids (n-3-PUFA) Can Prevent TBI-Induced Visual, Motor, and Emotional Deficits in Mice
Abstract
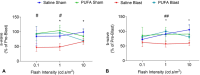
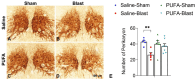
Traumatic brain injury (TBI) causes neuroinflammation and can generate long-term pathological consequences, including motor and visual impairments, cognitive deficits, and depression. In our previous study, we found that Fat1+-transgenic mice with higher endogenous n-3 polyunsaturated fatty acids (n-3 PUFA) were protected from post-TBI behavioral deficits and exhibited reduced levels of TBI-induced microglial activation, inflammatory factors, and sphingolipid ceramide, a lipid mediator of inflammation and cell death. This study's objective was to evaluate if feeding n-3 PUFA (EPA and docosahexaenoic acid, DHA 2:1) could restrict the elevation of ceramide in brain tissue and prevent TBI-mediated sensory-motor and behavioral deficits. Wildtype C57/BL6 mice were gavage pre-fed with PUFA (EPA: DHA = 2:1) at 500 mg/kg body weight/week for 2 weeks before and 4 weeks after exposure to left side focal cranial air-blast (50 psi) TBI or sham-blast (0-psi). Saline-gavaged mice served as controls. Following blast injury, various motor, visual, and behavioral tests were conducted, and brain tissues were collected for histological and biochemical assays. Lipidomics analysis confirmed a significant elevation of EPA in the plasma and brain tissue of PUFA-fed mice. TBI-Blast brain tissues were found to have elevated ceramide levels in control mice but not in PUFA-fed mice. Moreover, PUFA-fed mice demonstrated protection against motor impairment, photoreceptor dysfunction, depression, oculomotor nerve degeneration, and microglia activation in the optic tract. Our results demonstrate that EPA-mediated suppression of ceramide biosynthesis and neuroinflammatory factors in PUFA-fed mice is associated with significant protection against the visual, motor, and emotional deficits caused by TBI.
Keywords: Ceramide; Emotional deficits; N-3 PUFA; Neuroinflammation; Traumatic brain injury (TBI); Visual deficits.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval and Consent to Participate: Not applicable. Animals: Animal studies approved by the UTHSC IACUC committee. Approval # 19–0104. Consent for Publication: Not applicable. Author Consent: All the authors have seen the manuscript and approved it for publication. Competing interests: The authors declare no competing interests.
Figures






References
-
- Vos PE, Alekseenko Y, Battistin L, Ehler E, Gerstenbrand F, Muresanu DF et al (2012) Mild traumatic brain injury. Eur J Neurol 19(2):191–198 - PubMed
-
- Georges A, Das JM (2024) Traumatic brain injury (Archive). StatPearls. StatPearls Publishing Copyright © 2024. StatPearls Publishing LLC - PubMed
-
- Management of Concussion/mTBI Working Group (2009) VA/DoD Clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev 46(6):Cp1–68 - PubMed
MeSH terms
Substances
Grants and funding
- R01 EY031316/EY/NEI NIH HHS/United States
- W81XWH-20-1-0900/US Department of Defense ofce of the Congressionally Directed Medical Research Programs (CDMRP), Vision Research Program grant
- I01 BX004893/BX/BLRD VA/United States
- BX001792/US Veterans' Administration (VA Merit Review)
- I01 BX001792/BX/BLRD VA/United States
- EY022071/EY/NEI NIH HHS/United States
- IK6 BX004603/BX/BLRD VA/United States
- R01 AI139072/AI/NIAID NIH HHS/United States
- I01BX004893/US Veterans' Administration (VA Merit Review Award)
- R01 EY022071/EY/NEI NIH HHS/United States
- IK6BX004603/US Veterans' Administration Senior Research Career Scientist Award
- R01 GM137394/GM/NIGMS NIH HHS/United States
- R01 GM137578/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

