Hematologic and lymphatic disorders associated with chimeric antigen receptor T-cell therapy: a pharmacovigilance analysis of the FDA adverse event reporting system (FAERS) database
- PMID: 40346502
- PMCID: PMC12063233
- DOI: 10.1186/s12885-025-14227-4
Hematologic and lymphatic disorders associated with chimeric antigen receptor T-cell therapy: a pharmacovigilance analysis of the FDA adverse event reporting system (FAERS) database
Abstract
Background: As the application of Chimeric Antigen Receptor T-cell (CAR-T) therapy in cancer treatment becomes increasingly widespread, associated hematologic and lymphatic system adverse events pose significant challenges to its clinical use. Therefore, we aim to comprehensively investigate and summarize the hematologic and lymphatic system AEs associated with CAR-T therapy.
Methods: We extracted CAR-T-related adverse event reports from the FDA Adverse Event Reporting System (FAERS) database for the period from August 2017 to December 2023. Disproportionality analysis using the Reporting Odds Ratio (ROR) and Information Component (IC) was performed to identify CAR-T-associated hematologic and lymphatic system AEs. We employed LASSO regression analysis to identify hematologic and lymphatic system AEs associated with mortality.
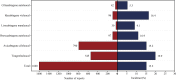
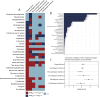
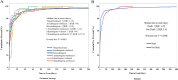
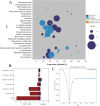
Results: In the FAERS database, we identified 1,600 individual case safety reports of hematologic and lymphatic system AEs related to CAR-T therapy. The median age of patients was 57 years (interquartile range [IQR] 32-67), with fatal outcomes in 15.3% of cases. We identified 25 significant adverse event signals associated with CAR-T therapy. B-cell aplasia (ROR025 = 1054.56, IC025 = 4.74), cytopenia (ROR025 = 17.27, IC025 = 3.81), hypofibrinogenemia (ROR025 = 100.18, IC025 = 2.46), anemia (ROR025 = 1.87, IC025 = 0.59), febrile bone marrow aplasia (ROR025 = 55.32, IC025 = 2.70), and pancytopenia (ROR025 = 7.18, IC025 = 1.42) were the most significant hematologic and lymphatic system AEs for tisa-cel, axi-cel, brexu-cel, liso-cel, ide-cel, and cilta-cel, respectively. Most hematologic and lymphatic system AEs occurred within 10 days post-CAR-T infusion. Hematologic and lymphatic system AEs were associated with a mortality rate of 15.3%. Our analysis revealed 15 hematologic and lymphatic system AEs closely associated with mortality in CAR-T-treated patients, including splenic hemorrhage, disseminated intravascular coagulation, and pancytopenia.
Conclusions: Our study found that hematologic and lymphatic system AEs were more closely associated with anti-CD19 CAR-T and CAR-T containing CD28. Splenic hemorrhage, disseminated intravascular coagulation, and pancytopenia were identified as hematologic and lymphatic system AEs that, while less frequently reported clinically, were highly associated with mortality.
Keywords: Adverse events; CAR-T therapy; FAERS; Hematologic and lymphatic system toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. FDA Adverse Event Reporting System is a spontaneous reporting system, the publicly available data are anonymized, and therefore, obtaining consent to participate is not applicable. The present pharmacovigilance study was conducted using a public database of spontaneous reports. Given the use of deidentified data, ethical approval was not considered necessary. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Safety assessment of anti-B cell maturation antigen chimeric antigen receptor T cell therapy: a real-world study based on the FDA adverse event reporting system database.Front Immunol. 2024 Sep 3;15:1433075. doi: 10.3389/fimmu.2024.1433075. eCollection 2024. Front Immunol. 2024. PMID: 39290710 Free PMC article.
-
Dermatologic Adverse Events Associated With Chimeric Antigen Receptor T-Cell Therapy: A Pharmacovigilance Analysis of the FDA Reporting System.Transplant Cell Ther. 2024 Oct;30(10):1035.e1-1035.e7. doi: 10.1016/j.jtct.2024.06.024. Epub 2024 Jun 28. Transplant Cell Ther. 2024. PMID: 38945480
-
Hemophagocytic lymphohistiocytosis and disseminated intravascular coagulation are underestimated, but fatal adverse events in chimeric antigen receptor T-cell therapy.Haematologica. 2023 Aug 1;108(8):2067-2079. doi: 10.3324/haematol.2022.281455. Haematologica. 2023. PMID: 36794498 Free PMC article.
-
Chimeric antigen receptor T-cells safety: A pharmacovigilance and meta-analysis study.Am J Hematol. 2021 Sep 1;96(9):1101-1111. doi: 10.1002/ajh.26259. Epub 2021 Jun 23. Am J Hematol. 2021. PMID: 34057232
-
Interstitial lung disease with antibody-drug conjugates: a real-world pharmacovigilance study based on the FAERS database during the period 2014-2023.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241299935. doi: 10.1177/17534666241299935. Ther Adv Respir Dis. 2024. PMID: 39660786 Free PMC article.
References
-
- Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–32. 10.1038/s41591-021-01622-0. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene Maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. 10.1016/S0140-6736(20)31366-0. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

