HIF-1α regulated GLUT1-mediated glycolysis enhances Treponema pallidum-induced cytokine responses
- PMID: 40346557
- PMCID: PMC12065375
- DOI: 10.1186/s12964-025-02211-1
HIF-1α regulated GLUT1-mediated glycolysis enhances Treponema pallidum-induced cytokine responses
Abstract
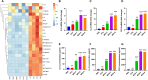
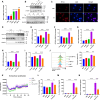
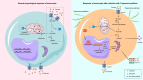
Syphilis, caused by Treponema pallidum (Tp), represents a significant public health challenge. The clinical manifestations of syphilis are attributed to local inflammatory responses induced by Tp, notably monocyte infiltration into local lesions and the secretion of inflammatory cytokines. However, the mechanisms driving cytokine production in response to Tp infection remain largely unknown. Given that increased glycolysis is associated with inflammatory responses, we aimed to investigate the role of glycolysis in Tp-induced secretion of inflammatory cytokines. In this study, we found that Tp promotes the secretion of inflammatory cytokines IL-6, IL-8, and CCL2 from monocytes while enhancing glycolysis through increased GLUT1 plasma membrane expression and glucose uptake. Importantly, inhibiting glycolysis and GLUT1 reduced the Tp-induced secretion of monocyte inflammatory cytokines. Additionally, Tp significantly increased HIF-1α expression and induced its nuclear translocation, thereby promoting glycolysis by upregulating the expression of GLUT1 and LDHA glycolytic enzymes. Knockdown of HIF-1α inhibits Tp-induced monocyte cytokine secretion, highlighting the crucial role of HIF-1α-mediated glycolysis in the cytokine response to Tp. Also, expression of HIF-1α and an increase in glycolysis were confirmed in patients with syphilis. In conclusion, we demonstrated that HIF-1α-regulated GLUT1-mediated glycolysis enhances inflammatory cytokine secretion following Tp infection. Our findings not only elucidate the mechanism of glycolysis in Tp-induced inflammatory responses in monocytes but also contribute to the development of a potential biomarker in syphilis diagnosis and treatment.
Keywords: Treponema pallidum; HIF-1α; Glycolysis; Inflammatory; Monocyte.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Triiodothyronine promotes the proliferation and chemoresistance of cholangiocarcinoma cells via HIF-1α/Glut1-stimulated glycolysis.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167814. doi: 10.1016/j.bbadis.2025.167814. Epub 2025 Mar 31. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40168755
-
Enhanced Aerobic Glycolysis by S-Nitrosoglutathione via HIF-1α Associated GLUT1/Aldolase A Axis in Human Endothelial Cells.J Cell Biochem. 2017 Aug;118(8):2443-2453. doi: 10.1002/jcb.25911. Epub 2017 Apr 25. J Cell Biochem. 2017. PMID: 28121054
-
Basic fibroblast growth factor regulates glucose metabolism through glucose transporter 1 induced by hypoxia-inducible factor-1α in adipocytes.Int J Biochem Cell Biol. 2011 Nov;43(11):1602-11. doi: 10.1016/j.biocel.2011.07.009. Epub 2011 Jul 26. Int J Biochem Cell Biol. 2011. PMID: 21810481
-
How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer?Ann Diagn Pathol. 2013 Jun;17(3):305-11. doi: 10.1016/j.anndiagpath.2012.12.002. Epub 2013 Feb 1. Ann Diagn Pathol. 2013. PMID: 23375385 Review.
-
HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms.Mini Rev Med Chem. 2009 Aug;9(9):1084-101. doi: 10.2174/138955709788922610. Mini Rev Med Chem. 2009. PMID: 19689405 Review.
References
-
- Liu Z, Zhang X, Xiong S, et al. Endothelial dysfunction of syphilis: Pathogenesis. J Eur Acad Dermatol Venereol. 2024. 10.1111/jdv.19899. - PubMed
-
- Bhugra P, Maiti A. Secondary Syphilis. N Engl J Med. 2020;383(14):1375. 10.1056/NEJMicm2001103. - PubMed
-
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: Historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82(1):1–14. 10.1016/j.jaad.2019.02.073. - PubMed
MeSH terms
Substances
Grants and funding
- nos. 81971980/National Natural Science Foundation of China
- no. 2019SK1010/Major Scientific and Technological Projects for collaborative prevention and control of birth defects in Hunan Province
- no. 2023JJ30530/Hunan Province Natural Science Foundation
- R2023004/Health High-Level Talents Major Scientific Research Project of Hunan Provincial Health Commission
- no. 20221064723/Health Research Key Project of Hunan Provincial Health Commission
LinkOut - more resources
Full Text Sources
Miscellaneous

