Time to administer polymyxin B hemoperfusion and hemodynamics in patients with septic shock requiring high-dose norepinephrine: a predetermined analysis of a prospective cohort study
- PMID: 40346574
- PMCID: PMC12063383
- DOI: 10.1186/s13054-025-05422-7
Time to administer polymyxin B hemoperfusion and hemodynamics in patients with septic shock requiring high-dose norepinephrine: a predetermined analysis of a prospective cohort study
Abstract
Background: Delayed administration of polymyxin B hemoperfusion (PMX-HP) for septic shock could diminish its efficacy in real-world clinical settings.
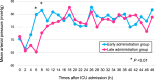
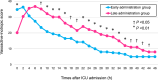
Methods: BEAT-SHOCK (BEst Available Treatment for septic SHOCK) registry is a prospective registry consisting of 309 adult patients with septic shock requiring high-dose norepinephrine (≥ 0.2 μg/kg/min). This predetermined analysis included 82 patients treated with PMX-HP. They were grouped according to the median time from intensive care unit (ICU) admission to administration of PMX-HP: the early administration group (n = 40) and the late administration group (n = 42). The primary outcome was short-term hemodynamic status, including mean arterial pressure and vasoactive-inotropic score (VIS; calculated from doses of dopamine, dobutamine, norepinephrine, epinephrine, vasopressin, milrinone, and levosimendan) within 48 h after ICU admission.
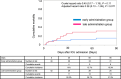
Results: The median time from ICU admission to administration of PMX-HP was 265 min (interquartile range [IQR]: 113-480). The median ages were 70 (IQR: 59-81) and 72 (IQR: 64-80) years (P = 0.77), and 21/40 (53%) and 25/42 (60%) patients were male (P = 0.52) in the early and late administration groups, respectively. The dose of norepinephrine at ICU admission was 0.33 (IQR: 0.24-0.47) and 0.30 (IQR: 0.22-0.34) μg/kg/min in the early and late administration groups, respectively (P = 0.17). Within 48 h after ICU admission, mean arterial pressure was significantly higher at 6 h and 8 h, and VIS was significantly lower at 8 h and thereafter in the early administration group. Within a 28-day period, there were 23 (IQR: 21-25) and 21 (IQR: 0-24) vasopressor/inotrope-free days (P = 0.027), and 18 (IQR: 1-23) and 14 (IQR: 0-19) ICU-free days (P = 0.025) in the early and late administration groups, respectively. The cumulative mortality at 90 days was 15.3% in the early administration group and 31.3% in the late administration group (adjusted hazard ratio 0.38; 95% confidence interval 0.13-1.09).
Conclusions: In patients with septic shock, early administration of PMX-HP was associated with higher mean arterial pressure and lower VIS within 48 h after ICU admission. Additionally, it may be associated with an improved clinical course, represented by more ICU-free and vasopressor/inotrope-free days. Trial registration UMIN Clinical Trial Registry on 1 November 2019 (registration no. UMIN000038302).
Keywords: Blood purification; Polymyxin B hemoperfusion; Septic shock.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The ethics committee of Tohoku University Hospital (Approval Number 2019-1-402) and the ethics committees of all other participating hospitals approved this study with an opt-out policy in accordance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects. Written informed consent was obtained from patients or their proxies to collect data about functional outcomes at 90 days by distributing questionnaires. This study was conducted in compliance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects and the principles of the Declaration of Helsinki. Consent for publication: The ethics committee of Tohoku University Hospital (approval number 2019-1-402) and the ethics committees of all other participating hospitals approved this study with an opt-out policy. Competing interests: Dr. Miyamoto reports lecturer's fees from Asahi Kasei Pharma, Japan Blood Products Organization, and Chugai Pharmaceutical. Dr. Kawazoe reports lecturer’s fees from Toray Industries Inc. and Asahi Kasei Pharma. Dr. Kyo reports lecturer's fee from TXP Medical. Dr. Masaki Takahashi reports lecture's fees from Japan Blood Products Organization and Eisai Co., Ltd.. Dr. Gaku Takahashi reports lecturer’s fees from Toray Industries Inc. and Asahi Kasei Pharma. and JB Pharma. Dr. Hanajima reports lecturer's fee from Toray Industries Inc.. Dr Morimoto reports lecturer's fees from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Pfizer, Tsumura and UCB; manuscript fee from Pfizer; advisory board for GlaxoSmithKline, Novartis and Teijin.
Figures

References
-
- Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:775–87. 10.1001/jama.2016.0289. - PMC - PubMed
-
- Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46:997–1000. 10.1097/ccm.0000000000003119. - PubMed
-
- Noritomi DT, Ranzani OT, Monteiro MB, Ferreira EM, Santos SR, Leibel F, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med. 2014;40:182–91. 10.1007/s00134-013-3131-5. - PubMed
-
- Houwink AP, Rijkenberg S, Bosman RJ, van der Voort PH. The association between lactate, mean arterial pressure, central venous oxygen saturation and peripheral temperature and mortality in severe sepsis: a retrospective cohort analysis. Crit Care. 2016;20:56. 10.1186/s13054-016-1243-3. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

