Plasma GFAP for populational enrichment of clinical trials in preclinical Alzheimer's disease
- PMID: 40346617
- PMCID: PMC12064411
- DOI: 10.1002/alz.70209
Plasma GFAP for populational enrichment of clinical trials in preclinical Alzheimer's disease
Abstract
Introduction: Cognitively unimpaired (CU) amyloid beta (Aβ)+ individuals with elevated plasma glial fibrillary acidic protein (GFAP) have an increased risk of Alzheimer's disease (AD)-related progression. We tested the utility of plasma GFAP for population enrichment CU populations in clinical trials.
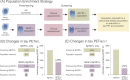
Methods: We estimated longitudinal progression, effect size, and costs of hypothetical clinical trials designed to test an estimated 25% drug effect on reducing tau positron emission tomography (PET) accumulation in the medial temporal lobe (MTL) and temporal neocortical region (NEO-T).
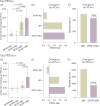
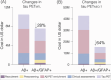
Results: CU GFAP+/Aβ+ individuals present an increased annual rate of change and effect size in tau PETMTL and tau PETNEO-T compared to the other groups. An enrichment strategy selecting CU GFAP+/Aβ+ individuals would require a smaller sample size (≈ 57% reduction) and fewer Aβ PET scans (≈ 74% reduction) than trials enriched with Aβ PET alone, reducing total clinical trial costs by up to 64%.
Discussion: Our results suggest that clinical trials focusing on preclinical AD recruiting Aβ+ individuals with elevated GFAP levels would improve cost effectiveness.
Highlights: Cognitively unimpaired (CU) glial fibrillary acidic protein (GFAP)+/amyloid beta (Aβ)+ shows increased changes in tau positron emission tomography (PET) . CU GFAP+/Aβ+ enriched clinical trials require a reduced sample size compared to Aβ+ only. CU GFAP+/Aβ+ enrichment reduces Aβ PET scans required and costs. CU GFAP+/Aβ+ enrichment allows the selection of individuals at early stages of the Alzheimer's disease continuum.
Keywords: clinical trial enrichment; glial fibrillary acidic protein; positron emission tomography imaging; preclinical Alzheimer's disease; tau deposition.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, reMYND, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and on advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. S.G. received consulting fees as a member of the scientific advisory boards in Abbvie, Alzheon, AmyriAD, Eisai, Enigma/Meilleur, Lilly, Okutsa, Novo Nordisk, TauRx; honoraria for educational videos from Lundbeck; and reimbursement for AD/PD 2024 travel expenses by TauRx. S.G. is a board member at the Sharon and Robert Francis Foundation, Toronto, Canada, and the Canadian Conference on Dementia (CCD). E.R.Z. has served on the scientific advisory board of Nintx, Novo Nordisk, and Masima. He is also a co‐founder and a minority shareholder at Masima. P.R‐N. has served on scientific advisory boards and/or as a consultant for Roche, Novo Nordisk, Eisai, and Cerveau radiopharmaceuticals. N.J.A. has given lectures in symposia sponsored by Lilly and Quanterix. J.T. has served as a consultant for the Neurotorium educational platform and for Alzheon Inc. P.V. has served on scientific advisory boards for Novo Nordisk, Eisai, and Lilly. G.T.B. receives salary and has stocks from Janssen R&D. S.C.J. serves on advisory boards for AlzPATH and Enigma Biomedical. The other authors declare that they have no conflict of interest. Author disclosures are available in the supporting information.
Figures

References
MeSH terms
Substances
Grants and funding
- R01 AG083874/AG/NIA NIH HHS/United States
- Aina (Ann) Wallströms
- Gamla Tjänarinnor Foundation
- #ALFGBG-715986/ALF-agreement
- AARFD-24-1313939/Fonds de Recherche du Québec-Santé
- 159815/CAPMC/ CIHR/Canada
- Mary-Ann Sjöbloms Foundation
- #ALFGBG-965240/ALF-agreement
- Swedish Dementia Foundation
- AARFD-22-923814/Fonds de Recherche du Québec-Santé
- #ALFGBG-71320/European Union's Horizon Europe research
- County Councils
- R01 AG075336/AG/NIA NIH HHS/United States
- #AF-930351/Swedish Alzheimer Foundation
- 5R01AG075336/National Institute in Aging
- 2020-VICO-279314/Chercheur Boursier
- Familjen Rönströms Stiftelse
- 312410/20182/Kirsten and Freddy Johansen Foundation
- 101053962/European Union's Horizon Europe research
- AG021155/Chercheur Boursier
- AG027161/Chercheur Boursier
- #AF-939721/Swedish Alzheimer Foundation
- #AF-930627/Alzheimerfonden
- #201809-2016862/European Union's Horizon Europe research
- AARFD-24-1307995/ALZ/Alzheimer's Association/United States
- #FO2017-0243/Swedish Alzheimer Foundation
- RFN 152985/CAPMC/ CIHR/Canada
- #2017-00915/Swedish Research Council
- 409066/2022-2/Kirsten and Freddy Johansen Foundation
- Swedish Parkinson Foundation
- #ADSF-21-831377-C/European Union's Horizon Europe research
- UKDRI-1003/National Institute of Health and Care Research
- #2021-03244/Instituto Serrapilheira
- Olav Thon Foundation
- #FO2020-0240/Hjärnfonden
- AARFD-22-974627/Fonds de Recherche du Québec-Santé
- CCNA; MOP-11-51-31-team 1/Canadian Consortium of Neurodegeneration and Aging
- R01 AG027161/AG/NIA NIH HHS/United States
- 312306/2021-0/Kirsten and Freddy Johansen Foundation
- #AARF-21-850325/Instituto Serrapilheira
- Serra-1912-31365/Instituto Serrapilheira
- #2020-00124/Agneta Prytz-Folkes & Gösta Folkes Foundation
- JPND2019-466-236/European Union Joint Program for Neurodegenerative Disorders
- #A2020812F/BrightFocus Foundation
- International Society for Neurochemistry's Career Development Grant
- #ADSF-21-831376-C/European Union's Horizon Europe research
- #FO2022-0270/Stiftelsen för Gamla Tjänarinnor
- R01 AG021155/AG/NIA NIH HHS/United States
- AG021155/AG/NIA NIH HHS/United States
- 2024-VICO-356138/Chercheur Boursier
- RF1 AG027161/AG/NIA NIH HHS/United States
- 2020-VICO-279314/Fonds de Recherche du Québec - Santé
- 435642/2018-9/Kirsten and Freddy Johansen Foundation
- 5R01AG073267/National Institute in Aging
- AARF-D-231150249/Instituto Serrapilheira
- MOP-11-51-31/CAPMC/ CIHR/Canada
- Weston Brain Institute
- #AF-994551/Swedish Alzheimer Foundation
- 24AARFD-1243899/Fonds de Recherche du Québec-Santé
- AARFD-24-1307995/Fonds de Recherche du Québec-Santé
- #2022-01018/Anna Lisa and Brother Björnsson's Foundation
- #AF-968270/Swedish Alzheimer Foundation
- #2019-02397/Anna Lisa and Brother Björnsson's Foundation
- 5 P01 AG025204-17/Fonds de Recherche du Québec-Santé
- #ADSF-24-1284328-C/European Union's Horizon Europe research
- 465671/2014-4/Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection
- Gun and Bertil Stohnes Foundation
- CFI Project 34874/Brain Canada Foundation
- #2023-00356/Anna Lisa and Brother Björnsson's Foundation
- Swedish government
- #ADSF-21-831381-C/European Union's Horizon Europe research
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- R01 AG073267/AG/NIA NIH HHS/United States
- 33397/Brain Canada Foundation
- #2022-00732/Swedish Research Council
- ALZ-NAN-22-928381/Instituto Serrapilheira
- P01 AG025204/AG/NIA NIH HHS/United States
- 162303/CAPMC/ CIHR/Canada
- SG-23-1038904 QC/European Union Joint Program for Neurodegenerative Disorders
- ZEN-21-848495/European Union Joint Program for Neurodegenerative Disorders
- #ALZ2022-0006/Swedish Alzheimer Foundation
- 2020-VICO-279314/Fonds de Recherche du Québec-Santé
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

