Keratin 6A promotes skin inflammation through JAK1-STAT3 activation in keratinocytes
- PMID: 40346694
- PMCID: PMC12065298
- DOI: 10.1186/s12929-025-01143-9
Keratin 6A promotes skin inflammation through JAK1-STAT3 activation in keratinocytes
Abstract
Background: Skin barrier dysfunction and immune activation are hallmarks of inflammatory skin diseases such as rosacea and psoriasis, suggesting shared pathogenic mechanisms. While barrier disruption may trigger or exacerbate skin inflammation, the precise underlying mechanisms remain unclear. Notably, epidermal barrier compromise leads to a marked increase in barrier alarmin expression. Among these, keratin 6A (KRT6A) plays a role in maintaining skin barrier integrity.
Methods: We treated mouse skin and human keratinocytes, with and without KRT6A expression, with LL37/TNF-α and assessed the severity of inflammation. The specific mechanism by which KRT6A promotes skin inflammation was investigated using mass spectrometry and immunoprecipitation assays.
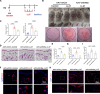
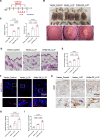
Results: KRT6A expression was elevated in lesional skin from patients and mouse models of rosacea and psoriasis. In mice with LL37-induced rosacea-like and imiquimod (IMQ)-induced psoriasis-like skin inflammation, KRT6A knockdown alleviated inflammation, whereas KRT6A overexpression exacerbated inflammatory responses. Mechanistically, KRT6A activated signal transducer and activator of transcription 3 (STAT3) and enhanced proinflammatory cytokine expression in keratinocytes by reducing Janus kinase 1 (JAK1) ubiquitination. This occurred through inhibition of ring finger protein 41 (RNF41)-mediated JAK1 binding.
Conclusions: Our findings indicate that KRT6A expression increases following epidermal barrier disruption and contributes to exacerbated skin inflammation in disease conditions. Targeting KRT6A may represent a novel therapeutic approach for inflammatory skin diseases associated with epidermal dysfunction.
Keywords: Inflammation; JAK1-STAT3; Keratin 6; Psoriasis; Rosacea.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the ethical committee of the Xiangya Hospital of Central South University (IRB number 202203076), and all subjects obtained written informed consent. All animals were purchased from Slack Company (Shanghai, China) and comply with the National Research Council’s Guide for the Care and Use of Laboratory Animals (IRB No. 2022020368). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Medgyesi B, Dajnoki Z, Béke G, Gáspár K, Szabó IL, Janka EA, et al. Rosacea is characterized by a profoundly diminished skin barrier. J Invest Dermatol. 2020;140(10):1938-50.e5. - PubMed
-
- Montero-Vilchez T, Segura-Fernández-Nogueras MV, Pérez-Rodríguez I, Soler-Gongora M, Martinez-Lopez A, Fernández-González A. Skin barrier function in psoriasis and atopic dermatitis: transepidermal water loss and temperature as useful tools to assess disease severity. J Clin Med. 2021. 10.3390/jcm10020359. - PMC - PubMed
-
- Gether L, Overgaard LK, Egeberg A. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–9. - PubMed
-
- Darlenski R, Kazandjieva J, Tsankov N, Fluhr JW. Acute irritant threshold correlates with barrier function, skin hydration and contact hypersensitivity in atopic dermatitis and rosacea. Exp Dermatol. 2013;22(11):752–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

