S100A9 as a potential novel target for experimental autoimmune cystitis and interstitial cystitis/bladder pain syndrome
- PMID: 40346703
- PMCID: PMC12065242
- DOI: 10.1186/s40364-025-00763-5
S100A9 as a potential novel target for experimental autoimmune cystitis and interstitial cystitis/bladder pain syndrome
Abstract
Background: Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic inflammatory disease of the bladder for which no effective therapy is currently available. Understanding the pathogenesis of IC/BPS and identifying effective intervention targets are of great clinical importance for its effective treatment. Our work focuses on elucidating the key targets and underlying mechanisms of IC/BPS.
Methods: We established an experimental autoimmune cystitis (EAC) mouse model and generated gene knockout mice to elucidate key mediators triggering chronic inflammatory damage in IC/BPS through using single-cell RNA sequencing, proteomic sequencing, and molecular biology experiments.
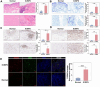
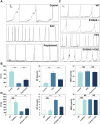
Results: Our study revealed that the infiltration and activation of macrophages, T cells, and mast cells exacerbated inflammatory bladder damage in both IC/BPS and EAC mice. Notably, cell-cell communication among bladder immune cells was significantly enhanced in EAC mice. Macrophages, as the main cell types altered in EAC mice, received and transmitted the most intensity signalling. Mechanistically, macrophages synthesized and secreted S100A9, which in turn facilitated macrophage polarization and promoted the production of pro-inflammatory cytokines. S100A9 emerged as an important pro-inflammatory and pathogenic molecule in IC/BPS and EAC. Further analysis demonstrated that S100A9 activation enhanced the inflammatory response and exacerbated bladder tissue damage in IC/BPS patients and EAC mice via TLR4/NF-κB and TLR4/p38 signalling pathways. Importantly, inhibition of S100A9 with paquinimod, as well as genetic knockout of S100A9, significantly attenuated the pathological process.
Conclusions: S100A9 is an important pro-inflammatory and pathogenic molecule in IC/BPS and EAC. Targeting S100A9-initiated signalling pathways may offer a novel therapeutic strategy for IC/BPS.
Keywords: Autoimmune disease; Experimental autoimmune cystitis; Inflammation; Interstitial cystitis/bladder pain syndrome; S100A9.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Research Council and Animal Care and Use Committee of the Army Military Medical University, China (Approval No.: AMUWEC2019414) and was conducted in accordance with animal welfare guidelines and the Declaration of Helsinki. Consent for publication: All authors agree to the publication of the article. Written informed consent for publication was obtained from all participants. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Elucidation of the pathophysiology of interstitial cystitis/bladder pain syndrome via experimental autoimmune cystitis rat model.Am J Physiol Regul Integr Comp Physiol. 2024 Aug 1;327(2):R250-R260. doi: 10.1152/ajpregu.00269.2023. Epub 2024 Jun 6. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38842512
-
The Effects of Neurokinin-1 Receptor Antagonist in an Experimental Autoimmune Cystitis Model Resembling Bladder Pain Syndrome/Interstitial Cystitis.Inflammation. 2019 Feb;42(1):246-254. doi: 10.1007/s10753-018-0888-2. Inflammation. 2019. PMID: 30196377
-
MiR-9-enriched mesenchymal stem cells derived exosomes prevent cystitis-induced bladder pain via suppressing TLR4/NLRP3 pathway in interstitial cystitis mice.Immun Inflamm Dis. 2024 Feb;12(2):e1140. doi: 10.1002/iid3.1140. Immun Inflamm Dis. 2024. PMID: 38415918 Free PMC article.
-
New insights into interstitial cystitis/bladder pain syndrome at single-cell resolution.BJUI Compass. 2025 Aug 4;6(8):e70051. doi: 10.1002/bco2.70051. eCollection 2025 Aug. BJUI Compass. 2025. PMID: 40765664 Free PMC article. Review.
-
Purinergic P2X7 receptors as therapeutic targets in interstitial cystitis/bladder pain syndrome; key role of ATP signaling in inflammation.Bladder (San Franc). 2019 Apr 8;6(1):e38. doi: 10.14440/bladder.2019.789. eCollection 2019. Bladder (San Franc). 2019. PMID: 32775480 Free PMC article. Review.
Cited by
-
The mechanism of plasma exosome miR-15a-5p targeting the CF-modified protein IGF1R to regulate alveolar epithelial autophagy and influence pulmonary interstitial fibrosis.Noncoding RNA Res. 2025 Jul 3;15:51-64. doi: 10.1016/j.ncrna.2025.07.001. eCollection 2025 Dec. Noncoding RNA Res. 2025. PMID: 40746334 Free PMC article.
References
-
- Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2022;208(1):34–42. - PubMed
-
- Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND interstitial cystitis epidemiology male study. J Urol. 2013;189(1):141–5. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

