Tau depletion diminishes vascular amyloid-related deficits in a mouse model of cerebral amyloid angiopathy
- PMID: 40346706
- PMCID: PMC12064415
- DOI: 10.1002/alz.70238
Tau depletion diminishes vascular amyloid-related deficits in a mouse model of cerebral amyloid angiopathy
Abstract
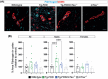
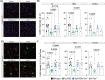
Introduction: Tau is essential for amyloid beta (Aβ)-induced synaptic and cognitive deficits in Alzheimer's disease (AD), making its downregulation a therapeutic target. Cerebral amyloid angiopathy (CAA), a major vascular contributor to cognitive decline, affects over 90% of patients with AD. This study explores the impact of tau downregulation on CAA pathogenesis.
Methods: We crossed the Familial Danish Dementia mouse model (Tg-FDD), which develops vascular amyloid, with tau-null (mTau-/-) mice to generate a CAA model lacking endogenous tau (Tg-FDD/mTau-/-). Behavioral, electrophysiological, histological, and transcriptomic analyses were performed.
Results: Tau depletion ameliorated motor and synaptic impairments, reduced vascular amyloid deposition, and prevented vascular damage. Tau ablation also mitigated astrocytic reactivity and neuroinflammation associated with vascular amyloid accumulation.
Conclusion: These findings provide the first in vivo evidence of the beneficial effects of tau downregulation in a CAA mouse model, supporting tau reduction as a potential therapeutic strategy for patients with parenchymal and vascular amyloid deposition.
Highlights: Tau ablation improves motor function and synaptic impair, reduces cerebrovascular amyloid deposits, and prevents vascular damage in a mouse model of cerebral amyloid angiopathy (CAA). Tau reduction decreases astrocytic reactivity, alters neuroinflammatory gene expression, and enhances oligodendrocyte function, suggesting a protective role against neuroinflammation in CAA. These findings highlight tau reduction as a potential therapeutic strategy to mitigate CAA-induced pathogenesis, with implications for treating patients with both parenchymal and vascular amyloid deposition.
Keywords: cerebral amyloid angiopathy; neuroinflammation; tau; vascular amyloid.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declared no conflict of interest in this study. Author disclosures are available in the Supporting Information.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

