VSIG4-Expressing Macrophages Contribute to Antiparasitic and Antimetastatic Responses in the Peritoneal Cavity
- PMID: 40346775
- PMCID: PMC12064866
- DOI: 10.1002/eji.202551804
VSIG4-Expressing Macrophages Contribute to Antiparasitic and Antimetastatic Responses in the Peritoneal Cavity
Abstract
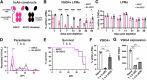
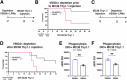
Large peritoneal macrophages (LPMs) play a role as gatekeepers of peritoneal homeostasis by providing a first line of defense against pathogens. A third of the LPMs express the surface receptor VSIG4, but it is unclear whether these cells differ from their VSIG4-negative counterparts and perform dedicated functions. We demonstrate that VSIG4+, but not VSIG4-, LPMs are in the majority derived from embryonal precursors, and their occurrence is largely independent of sex and microbiota but increases with age. Although their transcriptome and surface proteome are indistinguishable from VSIG4- LPMs at steady-state, VSIG4+ LPMs are superior in phagocytosing S. aureus bioparticles and colorectal carcinoma (CRC) cells. Anti-VSIG4 nanobody constructs that are ADCC-enabled allowed a selective elimination of the VSIG4+ LPM subset without affecting overall LPM abundance. This strategy uncovered a role for VSIG4+ LPMs in lowering the first peak of parasitemia in a Trypanosoma brucei brucei infection model and in reducing CRC outgrowth in the peritoneal cavity, a prime metastatic site in CRC patients. Altogether, our data uncover a protective role for VSIG4+ LPMs in infectious and oncological diseases in the peritoneal cavity.
Keywords: Trypanosoma brucei brucei infection; VSIG4; colorectal cancer metastasis; large peritoneal macrophage.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



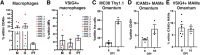
References
-
- Salm L., Shim R., Noskovicova N., and Kubes P., “Gata6+ Large Peritoneal Macrophages: An Evolutionarily Conserved Sentinel and Effector System for Infection and Injury,” Trends in Immunology 44, no. 2 (2023): 129–145. - PubMed
-
- Helmy K. Y., Katschke K. J., Gorgani N. N., et al., “CRIg: A Macrophage Complement Receptor Required for Phagocytosis of Circulating Pathogens,” Cell 124, no. 5 (2006): 915–927. - PubMed
-
- Zeng Z., Surewaard B. G. J., Wong C. H. Y., Geoghegan J. A., Jenne C. N., and Kubes P., “CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood‐Borne Gram‐Positive Bacteria,” Cell Host & Microbe 20, no. 1 (2016): 99–106. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

