The F-box protein CHALK10 mediates SEMIDWARF-1 ubiquitination and degradation to negatively regulate grain chalkiness in rice
- PMID: 40346802
- PMCID: PMC12281256
- DOI: 10.1016/j.xplc.2025.101354
The F-box protein CHALK10 mediates SEMIDWARF-1 ubiquitination and degradation to negatively regulate grain chalkiness in rice
Abstract
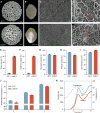
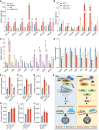
In rice (Oryza sativa), defects in endosperm development can give grains a chalky texture, which decreases grain quality and is thus undesirable for breeding and marketing. However, the molecular basis of chalkiness remains largely unknown. Here, we identified CHALK10, which encodes an F-box protein that negatively regulates rice chalkiness. The chalk10 mutant exhibited abnormal starch granule development, decreased starch content, and altered starch physicochemical properties compared with the wild type. CHALK10 interacts with the gibberellin (GA) oxidase SEMIDWARF-1 (SD1) and promotes the ubiquitination and degradation of SD1 through the 26S proteasome pathway. The grains of SD1-overexpressing plants exhibited increased chalkiness; introduction of the sd1 mutant allele into the chalk10 background largely suppressed the enhanced chalkiness observed in the chalk10 mutant. GA levels were higher in the chalk10 mutant than in the wild type, and application of the bioactive GA form GA3 increased grain chalkiness. The expression of genes related to starch degradation or biosynthesis was altered in the chalk10 mutant, resulting in reduced starch production and increased metabolizable sugar content in the endosperm. In summary, our findings reveal a novel regulatory mechanism of chalkiness and provide potential targets for improving rice quality.
Keywords: CHALK10; Oryza sativa; SD1; chalkiness; gibberellin; rice; starch metabolism.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Alabadí D., Gallego-Bartolomé J., Orlando L., García-Cárcel L., Rubio V., Martínez C., Frigerio M., Iglesias-Pedraz J.M., Espinosa A., Deng X.W., Blázquez M.A. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2007;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

