Progressive Thalamo-Cortical Disconnection in Amyotrophic Lateral Sclerosis Genotypes: Structural Degeneration and Network Dysfunction of Thalamus-Relayed Circuits
- PMID: 40346885
- PMCID: PMC12064938
- DOI: 10.1111/ene.70146
Progressive Thalamo-Cortical Disconnection in Amyotrophic Lateral Sclerosis Genotypes: Structural Degeneration and Network Dysfunction of Thalamus-Relayed Circuits
Abstract
Background: The thalamus is a key subcortical hub of numerous corticobasal and corticocortical circuits mediating a wealth of cognitive, behavioural, sensory and motor processes. While thalamic pathology is increasingly recognised in amyotrophic lateral sclerosis, its degeneration is often assessed in isolation instead of adopting a network-wise perspective and assessing the integrity of its rich cortical projections.
Methods: A prospective imaging study was conducted in a cohort of genetically stratified patients to assess the structural and functional integrity of thalamo-cortical circuits and volumetric alterations longitudinally.
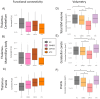
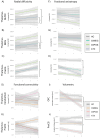
Results: The white matter integrity of thalamic projections to the anterior cingulate cortex, cerebellum, dorsolateral prefrontal cortex (DLPFC), Heschl's gyrus, medial frontal gyrus (MFG), orbitofrontal cortex, parietal cortex, postcentral gyrus and precentral gyrus (PreCG) is affected at baseline in ALS, which is more marked in C9orf72 hexanucleotide repeat carriers. Precentral gyrus and cerebellar grey matter volumes are also reduced, particularly in C9orf72. Longitudinal analyses capture progressive disconnection between the thalamus and frontal regions (DLPFC and MFG) in both C9orf72 positive and sporadic patients and progressive thalamo-PreCG disconnection in the sporadic C9orf72 negative cohort. Functional connectivity analyses revealed increasing thalamo-cerebellar connectivity in sporadic ALS and increasing thalamo-DLPFC connectivity in intermediate-length CAG repeat expansion carriers in ATXN2 over time.
Discussion: Our data provide evidence of extensive thalamo-cortical connectivity alterations in ALS. Corticobasal circuits mediating extrapyramidal, somatosensory, cognitive and behavioural functions are increasingly affected as the disease progresses. The degeneration of thalamic projections support the conceptualisation of ALS as a 'network disease' and the notion of 'what wires together degenerates together'.
Keywords: amyotrophic lateral sclerosis; magnetic resonance imaging; motor neuron disease; neuroimaging.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Westeneng H. J., Verstraete E., Walhout R., et al., “Subcortical Structures in Amyotrophic Lateral Sclerosis,” Neurobiology of Aging 36 (2015): 1075–1082. - PubMed
-
- Bede P., Omer T., Finegan E., et al., “Connectivity‐Based Characterisation of Subcortical Grey Matter Pathology in Frontotemporal Dementia and ALS: A Multimodal Neuroimaging Study,” Brain Imaging and Behavior 12 (2018): 1696–1707. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

