Optimal Analgesic Volume for Popliteal Plexus Block After Total Knee Arthroplasty: A Blinded RCT Protocol
- PMID: 40346895
- PMCID: PMC12065016
- DOI: 10.1111/aas.70057
Optimal Analgesic Volume for Popliteal Plexus Block After Total Knee Arthroplasty: A Blinded RCT Protocol
Abstract
Background: Adjunct treatment with a popliteal plexus block (PPB) provides moderate enhancement to multimodal analgesic regimens following total knee arthroplasty while maintaining motor function. However, the optimal anesthetic volume of local anesthetic for PPB remains unknown.
Aim: To compare the analgesic effects of PPB with 10 versus 20 mL of local anesthetics as an adjunct to a femoral triangle block after total knee arthroplasty.
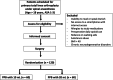
Methods: This blinded, controlled, randomized clinical trial will include 120 patients, randomly assigned to receive PPB with either 10 or 20 mL of bupivacaine 5 mg/mL. All patients undergo primary total knee arthroplasty under spinal anesthesia and receive a multimodal analgesia regimen, including paracetamol, ibuprofen, opioids, dexamethasone, and a femoral triangle block.
Outcomes: Primary outcome is 24-h postoperative opioid consumption. Secondary outcomes include the frequency of patients with opioid-free analgesia in the first 24 h after surgery, postoperative pain intensity at rest and during mobilization, postoperative muscle function of the leg, ability to mobilize with crutches 6 h after surgery, and Quality of Recovery-15 survey at 24 h after surgery.
Conclusion: This trial hopes to optimize postoperative pain management after total knee arthroplasty by providing valuable insights into the optimal analgesic volume for PPB.
Trial registration: Danish Data Protection Agency: 693807, clinicaltrials.gov: NCT06908837, CTIS: 2024-520204-26-00.
Keywords: peripheral nerve block; postoperative pain; regional anesthesia; total knee arthroplasty.
© 2025 The Author(s). Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Gerbershagen H. J., Aduckathil S., van Wijck A. J. M., Peelen L. M., Kalkman C. J., and Meissner W., “Pain Intensity on the First Day After Surgery,” Anesthesiology 118, no. 4 (2013): 934–944. - PubMed
-
- Kopp S. L., Børglum J., Buvanendran A., et al., “Anesthesia and Analgesia Practice Pathway Options for Total Knee Arthroplasty: An Evidence‐Based Review by the American and European Societies of Regional Anesthesia and Pain Medicine,” Regional Anesthesia and Pain Medicine 42 (2017): 683–697. - PubMed
-
- Memtsoudis S. G., Cozowicz C., Bekeris J., et al., “Peripheral Nerve Block Anesthesia/Analgesia for Patients Undergoing Primary Hip and Knee Arthroplasty: Recommendations From the International Consensus on Anesthesia‐Related Outcomes After Surgery (ICAROS) Group Based on a Systematic Review and Meta‐Analysis of Current Literature,” Regional Anesthesia and Pain Medicine 46, no. 11 (2021): 971–985. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical