Proton pump inhibitor concomitant use to prevent oxaliplatin-induced peripheral neuropathy: Clinical retrospective cohort study
- PMID: 40347077
- PMCID: PMC12309631
- DOI: 10.1002/phar.70028
Proton pump inhibitor concomitant use to prevent oxaliplatin-induced peripheral neuropathy: Clinical retrospective cohort study
Abstract
Background: Oxaliplatin-induced peripheral neuropathy (OIPN) is a major clinical challenge because it leads to discontinuation of chemotherapy. Omeprazole, a proton pump inhibitor (PPI), has been shown to prevent OIPN in a rat model. Therefore, we aimed to test whether the concomitant use of a PPI reduces oxaliplatin discontinuation due to OIPN.
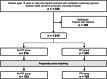
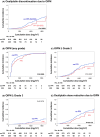
Methods: This retrospective study used data from 1015 patients who started treatment with oxaliplatin and evaluated two cohorts (PPI vs. non-PPI). The primary outcome measure was oxaliplatin discontinuation due to OIPN. A Kaplan-Meier curve was generated for cumulative doses and evaluated using the log-rank test and Cox proportional hazards analysis.
Results: The log-rank test showed that the number of patients who discontinued oxaliplatin due to OIPN was significantly lower in the PPI group (p = 0.0264). Cox proportional hazards analysis incorporated and analyzed factors previously reported as potentially affecting neuropathy (sex, age, use of PPIs, calcium channel antagonists, opioids and adjuvant analgesics, and the CAPOX [capecitabine + oxaliplatin] regimen). The analysis suggested that the concomitant use of PPIs was a factor in reducing oxaliplatin discontinuation (adjusted hazard ratio [HR] = 0.568, 95% confidence interval [CI], 0.344-0.937, p = 0.0269). Since there were significant differences in some patient demographics between the two groups, propensity score matching was performed to align the patient demographics and then reanalyzed. After propensity score matching, the same analysis as above showed that oxaliplatin discontinuation due to OIPN was significantly less common in the PPI group (p = 0.0081); cox proportional hazards analysis showed that PPI use was a factor that significantly reduced oxaliplatin discontinuation due to OIPN (adjusted HR = 0.478, 95% CI, 0.273-0.836, p = 0.0096).
Conclusions: These results suggest that concomitant PPI use may reduce oxaliplatin discontinuation due to OIPN in patients receiving oxaliplatin.
Keywords: drug repositioning; oxaliplatin; peripheral nerve injuries; proton pump inhibitors; retrospective studies.
© 2025 The Author(s). Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy published by Wiley Periodicals LLC on behalf of ACCP Foundation, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

