Targeted antineoplastic therapy in critically ill cancer patients: a multicenter analysis of the iCHOP registry
- PMID: 40347248
- PMCID: PMC12141367
- DOI: 10.1007/s00277-025-06400-3
Targeted antineoplastic therapy in critically ill cancer patients: a multicenter analysis of the iCHOP registry
Abstract
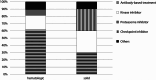
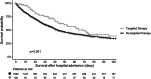
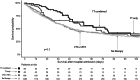
Approximately 20% of intensive care unit (ICU) patients have cancer, and their prognosis has markedly improved in recent years. In addition to improved treatment in the ICU, this is a result of advancements in cancer therapies, including the use of targeted therapies (TTs), such as antibodies and small-molecule kinase inhibitors. Despite the increasing use of TT, there are currently no comprehensive studies examining critically ill cancer patients receiving TT in the ICU. We studied the clinical characteristics of a multicenter cohort of cancer patients who received TT in the ICU. To this end, we extracted data from the iCHOP Registry, comprising critically ill cancer patients from nine centers in Germany and Austria, and analyzed patient characteristics, cancer therapies, and survival outcomes. We then employed Cox proportional hazards regression and Kaplan‒Meier survival analyses to explore factors associated with mortality. Of the 1,762 cancer patients admitted to the ICU who were analyzed for this study, 106 patients (6%) received TT in the ICU, such as antibody-based treatments, kinase inhibitors and proteasome inhibitors. Although the TT recipients were younger, there were several pronounced high-risk features in the TT cohort, as indicated by a greater proportion of hematologic malignancies and autologous stem cell transplantation (SCT), a greater percentage of progressive disease and fewer patients in complete remission at ICU admission than in patients not receiving TT in the ICU. Despite these more pronounced risk features, TT patients had a slightly longer median OS than did the other patients according to Kaplan‒Meier analysis. The factors associated with mortality according to Cox proportional hazards regression analysis included advanced directives, disease progression, SOFA score, invasive mechanical ventilation (IMV), renal replacement therapy, and duration of ICU and hospital stay. Critically ill cancer patients receiving TT in the ICU had distinct characteristics but had comparable survival outcomes compared to patients receiving any other or no antineoplastic therapy in the ICU. While disease status at ICU admission remains crucial, the present study indicates the feasibility and potential benefits of TT in selected ICU patients.
Keywords: Cancer; Immunotherapy; Intensive care; Targeted therapy; Toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review of the Medical Faculty and the University Hospital Cologne on December 18, 2013 (#13–380). All participating providers from the iCHOP registry obtained institutional review board approval from their institutions in accordance with local ethics regulations. Consent for publication: Not applicable. Competing interests: P.S. has received honoraria from Abbvie, Astro Pharma, Gilead, Jazz Pharmaceuticals, Novartis, Pfizer, and Roche; his University has received research funding from Amgen, Astellas, Astro Pharma, and Baxter; as well as competitive research grants from the Initiative Krebsforschung, the European Society of Intensive Care Medicine, and the European Commission. C.L. has received honoraria from Gilead. A.T. is an advisor for and reports receiving travel support from Kite/Gilead. J.G.B. has received research funding and travel support from Kite/Gilead. D.A.E. has received honoraria from Sanofi-Genzyme and Takeda. B.B. has received honoraria and research funding from Amgen, Gilead, MSD, Miltenyi, Noscendo, Novartis, Pfizer and Takeda. The remaining authors have no financial or non-financial interests to disclose.
Figures
Similar articles
-
Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit.Cochrane Database Syst Rev. 2018 Mar 27;3(3):CD010754. doi: 10.1002/14651858.CD010754.pub2. Cochrane Database Syst Rev. 2018. PMID: 29582429 Free PMC article.
-
Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia.Cochrane Database Syst Rev. 2016 Oct 25;10(10):CD008367. doi: 10.1002/14651858.CD008367.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Dec 24;12:CD008367. doi: 10.1002/14651858.CD008367.pub4. PMID: 27778318 Free PMC article. Updated.
-
Early versus late tracheostomy in critically ill COVID-19 patients.Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD015532. doi: 10.1002/14651858.CD015532. Cochrane Database Syst Rev. 2023. PMID: 37982427 Free PMC article.
-
Automated monitoring compared to standard care for the early detection of sepsis in critically ill patients.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD012404. doi: 10.1002/14651858.CD012404.pub2. Cochrane Database Syst Rev. 2018. PMID: 29938790 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Kochanek M, Shimabukuro-Vornhagen A, Russ K, Beutel G, Lueck C, Kiehl M et al (2020) [Prevalence of cancer patients in German intensive care units]. Med Klin Intensivmed Notfmed 115(4):312–319 - PubMed
-
- Soares M, Bozza FA, Azevedo LC, Silva UV, Correa TD, Colombari F et al (2016) Effects of organizational characteristics on outcomes and resource use in patients with Cancer admitted to intensive care units. J Clin Oncol 34(27):3315–3324 - PubMed
-
- Azoulay E, Schellongowski P, Darmon M, Bauer PR, Benoit D, Depuydt P et al (2017) The intensive care medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med 43(9):1366–1382 - PubMed
-
- Shimabukuro-Vornhagen A, Boll B, Kochanek M, Azoulay E, von Bergwelt-Baildon MS (2016) Critical care of patients with cancer. CA Cancer J Clin 66(6):496–517 - PubMed
-
- Shimabukuro-Vornhagen A, Boll B, Schellongowski P, Valade S, Metaxa V, Azoulay E et al (2022) Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J Clin 72(1):78–93 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

