T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination
- PMID: 40348752
- PMCID: PMC12065855
- DOI: 10.1038/s41467-025-59370-5
T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination
Abstract
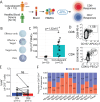
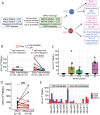
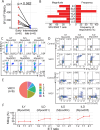
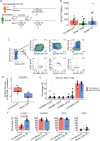
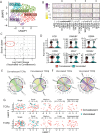
In 2022, a global mpox outbreak occurred, and remains a concern today. The T cell memory response to MPXV (monkeypox virus) infection has not been fully investigated. In this study, we evaluate this response in convalescent and MVA-BN (Modified Vaccinia Ankara - Bavarian Nordic) vaccinated individuals using VACV-infected cells. Strong CD8+ and CD4+ T cell responses are observed, and T cell responses are biased towards viral early expressed proteins. We identify seven immunodominant HLA-A*02:01 restricted MPXV-specific epitopes and focus our detailed phenotypic and scRNAseq analysis on the immunodominant HLA-A*02:01-G5R18-26-specific CD8+ T cell response. While tetramer+CD8+ T cells share similar differentiation and activation phenotypes, T cells from convalescent individuals show greater cytotoxicity, migratory potential to site of infection and TCR clonal expansion. Our data suggest that effective functional profiles of MPXV-specific memory T cells induced by Mpox infection may have an implication on the long-term protective responses to future infection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- WHO. Fifth Meeting of the International Health Regulations (2005) (IHR) (Emergency Committee on the Multi-country Outbreak of mpox (monkeypox), 2005).
-
- WHO. 2022–23 Mpox (Monkeypox) Outbreak: Global Trends (WHO, 2024).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

