RNA transcripts serve as a template for double-strand break repair in human cells
- PMID: 40348775
- PMCID: PMC12065846
- DOI: 10.1038/s41467-025-59510-x
RNA transcripts serve as a template for double-strand break repair in human cells
Abstract
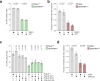
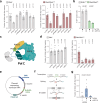
Double-strand breaks (DSBs) are toxic lesions that lead to genome instability. While canonical DSB repair pathways typically operate independently of RNA, growing evidence suggests that RNA:DNA hybrids and nearby transcripts can influence repair outcomes. However, whether transcript RNA can directly serve as a template for DSB repair in human cells remains unclear. In this study, we develop fluorescence and sequencing-based assays to show that RNA-containing oligonucleotides and messenger RNA can serve as templates during DSB repair. We conduct a CRISPR/Cas9-based genetic screen to identify factors that promote RNA-templated DSB repair (RT-DSBR). Of the candidate polymerases, we identify DNA polymerase zeta (Polζ) as a potential reverse transcriptase that facilitates RT-DSBR. Furthermore, analysis of cancer genome sequencing data reveals whole intron deletions - a distinct genomic signature of RT-DSBR that occurs when spliced mRNA guides repair. Altogether, our findings highlight RT-DSBR as an alternative pathway for repairing DSBs in transcribed genes, with potential mutagenic consequences.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.S. is a co-founder, consultant, and shareholder for REPARE Therapeutics. S.N.P is a consultant for AstraZeneca, Varian Medical Systems, and Philips. J.S.R.-F. reports current employment at AstraZeneca and stocks in AstraZeneca, Repare Therapeutics, Paige.AI; J.S.R.-F. previously held a fiduciary role in Grupo Oncoclinicas and consulted with Goldman Sachs Merchant Banking, Bain Capital, Repare Therapeutics, Paige.AI, Volition Rx and MultiplexDx. P.C.B. sits on the Scientific Advisory Boards of Intersect Diagnostics Inc., BioSymetrics Inc., and Sage Bionetworks. The remaining authors declare no competing interests.
Figures




Update of
-
RNA Transcripts Serve as a Template for Double-Strand Break Repair in Human Cells.bioRxiv [Preprint]. 2025 Feb 25:2025.02.23.639725. doi: 10.1101/2025.02.23.639725. bioRxiv. 2025. Update in: Nat Commun. 2025 May 10;16(1):4349. doi: 10.1038/s41467-025-59510-x. PMID: 40060534 Free PMC article. Updated. Preprint.
Similar articles
-
RNA Transcripts Serve as a Template for Double-Strand Break Repair in Human Cells.bioRxiv [Preprint]. 2025 Feb 25:2025.02.23.639725. doi: 10.1101/2025.02.23.639725. bioRxiv. 2025. Update in: Nat Commun. 2025 May 10;16(1):4349. doi: 10.1038/s41467-025-59510-x. PMID: 40060534 Free PMC article. Updated. Preprint.
-
Single-Strand Annealing Plays a Major Role in Double-Strand DNA Break Repair following CRISPR-Cas9 Cleavage in Leishmania.mSphere. 2019 Aug 21;4(4):e00408-19. doi: 10.1128/mSphere.00408-19. mSphere. 2019. PMID: 31434745 Free PMC article.
-
Human DNA polymerase η promotes RNA-templated error-free repair of DNA double-strand breaks.J Biol Chem. 2023 Mar;299(3):102991. doi: 10.1016/j.jbc.2023.102991. Epub 2023 Feb 8. J Biol Chem. 2023. PMID: 36758800 Free PMC article.
-
RNA-directed repair of DNA double-strand breaks.DNA Repair (Amst). 2015 Aug;32:82-85. doi: 10.1016/j.dnarep.2015.04.017. Epub 2015 May 1. DNA Repair (Amst). 2015. PMID: 25960340 Review.
-
Mapping cellular responses to DNA double-strand breaks using CRISPR technologies.Trends Genet. 2023 Jul;39(7):560-574. doi: 10.1016/j.tig.2023.02.015. Epub 2023 Mar 25. Trends Genet. 2023. PMID: 36967246 Free PMC article. Review.
References
-
- Pankotai, T., Bonhomme, C., Chen, D. & Soutoglou, E. DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks. Nat. Struct. Mol. Biol.19, 276–282 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

