NLRP3 inflammasome inhibits mitophagy during the progression of temporal lobe epilepsy
- PMID: 40348802
- PMCID: PMC12065917
- DOI: 10.1038/s41598-025-01087-y
NLRP3 inflammasome inhibits mitophagy during the progression of temporal lobe epilepsy
Abstract
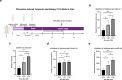
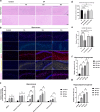
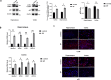
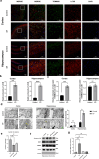
Epilepsy is a neurological disorder involving mitochondrial dysfunction and neuroinflammation. This study examines the relationship between NLRP3 inflammasome activation and mitophagy in the temporal lobe epilepsy, which has not been reported before. A pilocarpine-induced epileptic rat model was used to assess seizure activity and neuronal loss. Pyroptosis markers (NLRP3, cleaved Gasdermin D, IL-1β/IL-18), and autophagy/mitophagy activity (LC3B-II/I, BNIP3, TOMM20/LC3B colocalization) were analyzed via immunofluorescence, Western blot, and transmission electron microscopy. NLRP3 inhibitors and anti-IL-1β antibodies were administered to evaluate therapeutic effects. Epileptic rats exhibited progressive neuronal loss and seizure aggravation, correlating with NLRP3 inflammasome activation and pyroptosis. While general autophagy was upregulated, mitophagy was selectively impaired in the hippocampus. NLRP3 activation promoted IL-1β release, which suppressed mitophagy via PPTC7 upregulation. NLRP3 activation inhibitor (MCC950) and anti-IL-1β treatment restored mitophagy and reduced seizures. NLRP3 inflammasome-driven pyroptosis exacerbates epilepsy by impairing mitophagy activity via IL-1β/PPTC7. Targeted NLRP3 inhibition mitigates this cascade, offering a promising strategy for refractory epilepsy.
Keywords: Autophagy; Mitophagy; NLRP3 inflammasome; Pyroptosis; Temporal lobe epilepsy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The animal study was approved by the Animal Ethics Committee of Fujian Provincial Hospital, and all experimental procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The authors complied with the ARRIVE guidelines. Competing interests: The authors declare no competing interests.
Figures

References
-
- Dong, Y. et al. Ascorbic acid ameliorates seizures and brain damage in rats through inhibiting autophagy. - PubMed
MeSH terms
Substances
Grants and funding
- 2021J05075/the Natural Science Foundation of Fujian Province, China
- 2021J05075/the Natural Science Foundation of Fujian Province, China
- 2021J05075/the Natural Science Foundation of Fujian Province, China
- 2023Y9286/Joint Funds for the innovation of science and Technology, Fujian province
- 2024J011004/Fujian Provincial Natural Science Foundation
LinkOut - more resources
Full Text Sources

