Ubiquitin-activating enzyme1 (TgUAE1) acts as a key regulator of Toxoplasma gondii lytic cycle and homeostasis
- PMID: 40348811
- PMCID: PMC12065787
- DOI: 10.1038/s42003-025-08149-x
Ubiquitin-activating enzyme1 (TgUAE1) acts as a key regulator of Toxoplasma gondii lytic cycle and homeostasis
Abstract
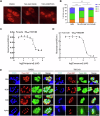
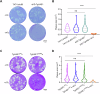
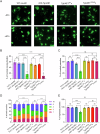
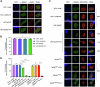
Ubiquitylation, regulated by the ubiquitin-proteasome system (UPS), is crucial for cell division and cycle transitions in Toxoplasma gondii. However, the primary E1 ubiquitin-activating enzyme (UAE1) in this process has been unclear. This study identified and characterized TGGT1_290290 (TgUAE1) as the canonical E1 enzyme in T. gondii. Through a combination of bioinformatics, biochemical, pharmacological, and genetic approaches, TgUAE1 was shown to exhibit typical E1 activity, particularly in forming K48- and K63-linked polyubiquitin chains. TAK-243, a UAE1 inhibitor, can effectively inhibit the ubiquitin pathway in T. gondii, as thermal stabilization experiments identified TgUAE1 as its intracellular target. Disruption of TgUAE1 severely impaired parasite homeostasis and suppressed the lytic cycle, highlighting its critical role in T. gondii fitness. Mutation of C634 in TgUAE1 confirmed that its enzymatic activity is essential for function. Transcriptomics and quantitative ubiquitin proteomics revealed TgUAE1 as a key regulator of the ubiquitination process and the broader gene expression network in T. gondii. These findings not only underscore the indispensable role of TgUAE1 in the life cycle of T. gondii but also offer valuable data that could inform future studies on parasite biology and the development of novel therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declared no competing interests.
Figures






References
-
- Dubey, J. P. Toxoplasmosis of Animals and Humans. (Toxoplasmosis of Animals and Humans, 2016).
-
- Gao, J. M. et al. Genetic analyses of Chinese isolates of Toxoplasma gondii reveal a new genotype with high virulence to murine hosts. Vet. Parasitol.241, 52–60 (2017). - PubMed
-
- Dubey, J. P. Toxoplasmosis in pigs-The last 20 years. Vet. Parasitol.164, 89–103 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

