Multi-parametric quantitative evaluation of murine cervical remodeling during pregnancy and postpartum
- PMID: 40348833
- PMCID: PMC12065793
- DOI: 10.1038/s41598-025-98765-8
Multi-parametric quantitative evaluation of murine cervical remodeling during pregnancy and postpartum
Abstract
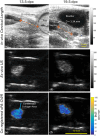
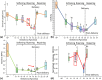
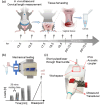
Cervical remodeling during pregnancy is a critical process that, if untimely, can lead to complications such as preterm birth (PTB). This study introduces a novel multi-parametric approach combining non-invasive imaging modalities to quantify cervical tissue changes during pregnancy and postpartum in a murine model. By integrating ultrasound-based measurements of cervical length, photoacoustic imaging of the collagen-to-water ratio, and elastography for tissue elasticity alongside histological assessments, this method provides a comprehensive evaluation of cervical remodeling. The findings reveal that combining these parameters significantly improves the accuracy of gestational age prediction compared to individual measurements, with a tri-parametric model achieving 85.3% prediction accuracy compared to 65.4% accuracy with histological analysis alone. This approach not only enhances the understanding of cervical remodeling but also holds potential as a minimally invasive, point-of-care diagnostic tool for early detection of cervical ripening and PTB risk. Ultimately, these advancements could inform clinical strategies for pregnancy management and labor induction, improving maternal and neonatal outcomes.
Keywords: Biomarkers; Cervical remodeling; Elasticity; Gestational age; Non-invasive; Photoacoustic; Ultrasound.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Lamont, R. F. et al. Commentary on a combined approach to the problem of developing biomarkers for the prediction of spontaneous preterm labor that leads to preterm birth, (in English). Placenta98, 13–23. 10.1016/j.placenta.2020.05.007 (2020). - PubMed

