Mobile health intervention CanRelax reduces distress in people with cancer in a randomized controlled trial
- PMID: 40348854
- PMCID: PMC12065826
- DOI: 10.1038/s41746-025-01688-x
Mobile health intervention CanRelax reduces distress in people with cancer in a randomized controlled trial
Abstract
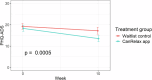
Mindfulness and relaxation exercises are effective face-to-face interventions for reducing distress in people with cancer. Their effectiveness in mobile health settings has yet to be investigated. This study evaluated the effectiveness of the CanRelax 2 app in reducing distress in people with cancer. German-speaking adults diagnosed with cancer within the last five years with elevated distress levels (Distress Thermometer ≥5) were recruited. Participants were randomized to the CanRelax 2 app or a waitlist control group. The primary endpoint was the Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS) after 10 weeks (210 participants). We observed a clinically meaningful larger reduction in PHQ-ADS scores in the intervention group compared to the control group (-3.7, 95%-CI from -5.7 to -1.6; p = 0.0005). Similar effects were found for distress, well-being, and self-regulation. Our results confirm the effectiveness of a mobile health app in reducing distress in people with cancer. Registration: German Clinical Trials Register (DRKS00027546) on 23.02.2022.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.M.W. has active research grants to the University for digital health projects from the DIZH, the Swiss Cancer Research foundation, the German health care Innovation Fund, and Newsense Lab GmbH and has received honoraria from Swiss hospitals for scientific presentations on the digitalization and AI in medicine. F.S. is founder of Byldr, which is company for developing mobile health solutions. During the development of CanRelax 2, T.K., F.S., and P.S. were developers and promoters of the open-source software platform MobileCoach. T.K. and P.S. are affiliated with the Centre for Digital Health Interventions (CDHI), a joint initiative of the Institute for Implementation Science in Health Care, University of Zurich, the Department of Management, Technology, and Economics at ETH Zurich, and the Institute of Technology Management and School of Medicine at the University of St.Gallen. C.D.H.I. is funded in part by CSS, a Swiss health insurer, MTIP, a Swiss digital health investor company, and Mavie Next, an Austrian health provider. T.K. was also a co-founder of Pathmate Technologies, a University spin-off company that creates and delivers digital clinical pathways. However, Pathmate Technologies, CSS, MTIP, and Mavie Next were not involved in this research. J.B. received honoraria for workshops on digital health. M.E. received institutional research grants from Kaiku Health and reports grants from Bristol Myers Squibb, Roche and institutional fees as a Scientific Advisory Board Member/Consultant from Roche, outside the submitted work. The remaining authors have no conflicts of interest to declare.
Figures

References
-
- Haller, H. et al. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncologica56, 1665–1676 (2017). - PubMed
-
- Lin, L.-Y. et al. Effects of mindfulness-based therapy for cancer patients: A systematic review and meta-analysis. J. Clin. Psychol. Med. Settings29, 432–445 (2022). - PubMed
LinkOut - more resources
Full Text Sources

