Molecular basis of TRPV3 channel blockade by intracellular polyamines
- PMID: 40348873
- PMCID: PMC12065880
- DOI: 10.1038/s42003-025-08103-x
Molecular basis of TRPV3 channel blockade by intracellular polyamines
Abstract
ThermoTRPV1-4 channels are involved in the regulation of multiple physiological processes, including thermo- and pain perception, thermoregulation, itch, and nociception and therefore tight control of their activity is a critical requirement for correct perception of noxious stimuli and pain. We previously reported a voltage-dependent inhibition of TRPV1-4 channels by intracellular polyamines that could be explained by high affinity spermine binding in, and passage through, the permeation path. Here, using electrophysiology and cryo-electron microscopy, we elucidate molecular details of TRPV3 blockade by endogenous spermine and its analog NASPM. We identify a high-affinity polyamine interaction site at the intracellular side of the pore, formed by residues E679 and E682, with no significant contribution of residues at the channel selectivity filter. A cryo-EM structure of TRPV3 in the presence of NASPM reveals conformational changes coupled to polyamine blockade. Paradoxically, although the TRPV3 'gating switch' is in the 'activated' configuration, the pore is closed at both gates. A modified blocking model, in which spermine interacts with the cytoplasmic entrance to the channel, from which spermine may permeate, or cause closure of the channel, provides a unifying explanation for electrophysiological and structural data and furnishes the essential background for further exploitation of this regulatory process.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




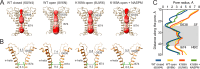

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

