Comparative efficacy of antidepressant medication for adolescent depression: a network meta-analysis and systematic review
- PMID: 40349006
- PMCID: PMC12065250
- DOI: 10.1186/s12888-025-06941-x
Comparative efficacy of antidepressant medication for adolescent depression: a network meta-analysis and systematic review
Abstract
Purpose: To evaluate the success rate of different antidepressants in addressing depression among teenagers, while also offering substantiation for the efficacy and tolerability of these treatments in this demographic.
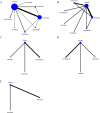
Methods: Participants were adolescents aged 6-18 years diagnosed with major depressive disorder according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) , Chinese Classification of Mental Disorders (CCMD-3) or equivalent diagnostic criteria (e.g., Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition(DSM-4) , International Classification of Diseases, Tenth/Eleventh Revision(ICD10/11) ) . We conducted a systematic search of major databases (PubMed, Cochrane Library, and Web of Science) for randomized controlled trials (RCTs) published up to October 2024. The search strategy included the following keywords: "Depression," "Depressive Disorders," "Emotional Disorders," "adolescent," "young adult, " "minors," "fluoxetine," "sertraline," "paroxetine," "agomelatine," "vilazodone," "escitalopram," and "venlafaxine."
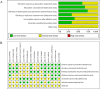
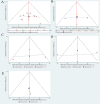
Results: Our network meta-analysis(NMA) included 15 RCTs involving 12,258 participants. The included studies were assessed using the Cochrane risk of bias tool. The majority of studies had low risk of bias in terms of randomization and allocation concealment, while some studies had unclear implementation of blinding or outcome assessment. The NMA results showed that in several major indicators Children's Depression Rating Scale-Revised (CDRS-R) , Clinical Global Impression-Severity (CGI-S) and Children's Global Assessment Scale (CGAS) , agomelatine (MD = -0.34, 95 % CI = -0.59, -0.09), fluoxetine (MD = -0.31, 95 % CI = -0.42, -0.21), sertraline (MD = -0.27, 95 % CI = -0.47, -0.06) were significantly better than placebo in improving CDRS-R. In terms of CGI-S, sertraline (MD = -4.39, 95 % CI = -4.77, -4.01) was more effective. In contrast to the placebo, escitalopram (MD = 2.08,95 % CI = 1.33,2.84) was more effective in CGAS; Surface Under the Cumulative Ranking Curve (SUCRA) values showed that escitalopram (96.1 % and 86.4 %) could achieve better therapeutic effects in CGAS and Clinical Global Impressions-Improvement (CGI-I) , and agomelatine (86.4 %) was more effective in improving CDRS-R scores than other drugs. Sertraline (100 %) appears to be the most likely strategy to decelerate the increase in CGI-I scores. The effectiveness of paroxetine (99.9%) in the management of Montgomery-Asberg Depression Rating Scale (MADRS) was significantly better than that of several other drugs.
Conclusion: For symptom severity scales, agomelatine (CDRS-R: SUCRA 86.4%) and paroxetine (MADRS: SUCRA 99.9%) demonstrated the greatest efficacy. For functional improvement, escitalopram ranked highest on CGAS (SUCRA 96.1%). Sertraline showed superiority in clinician-rated severity (CGI-S: SUCRA 100%) and improvement (CGI-I: SUCRA 80.2%). Clinical decisions should prioritize escitalopram for functional recovery and sertraline for severe cases requiring rapid symptom reduction.
Trial registration: PROSPERO registration number: CRD42024609880.
Keywords: Adolescents; Depression; Drug efficacy; Drug therapy; Network meta-analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This is a systematic review and meta-analysis, ethics approval and consent to participate are not applicable. Consent for publication: Not applicable. This study does not involve human participants. Competing interests: The authors declare no competing interests.
Figures


References
-
- Weavers B, Heron J, Thapar AK, Stephens A, Lennon J, Bevan Jones R, Eyre O, Anney RJL, Collishaw S, Thapar A, Rice F. The antecedents and outcomes of persistent and remitting adolescent depressive symptom trajectories: a longitudinal, population-based English study. Lancet Psychiatry. 2021;8(12):1053–61. - PubMed
-
- Yang CH, Lv JJ, Kong XM, Chu F, Li ZB, Lu W, Li XY. Global, regional and national burdens of depression in adolescents and young adults aged 10–24 years, from 1990 to 2019: findings from the 2019 Global Burden of Disease study. Br J Psychiatry. 2024;225(2):311–20. - PubMed
-
- Zhou X, Teng T, Del Giovane C, Furukawa TA, Weisz JR, Cipriani A, Xie P. Treatment of depression in children and adolescents – Authors’ reply. Lancet Psychiatry. 2021;8(2):97–8. - PubMed
-
- Hazell P. Updates in treatment of depression in children and adolescents. Curr Opin Psychiatry. 2021;34(6):593–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

