Intrapleural dual blockade of IL-6 and PD-L1 reprograms CAF dynamics and the tumor microenvironment in lung cancer-associated malignant pleural effusion
- PMID: 40349069
- PMCID: PMC12065214
- DOI: 10.1186/s12931-025-03263-0
Intrapleural dual blockade of IL-6 and PD-L1 reprograms CAF dynamics and the tumor microenvironment in lung cancer-associated malignant pleural effusion
Abstract
Background: Malignant pleural effusion (MPE) is a severe complication in lung cancer, characterized by an immunosuppressive tumor microenvironment (TME) and limited therapeutic options. This study investigates the role of IL-6 in regulating immune suppression and tumor progression in MPE and evaluates the efficacy of dual IL-6 and PD-L1 blockade.
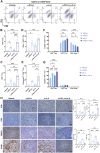
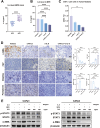
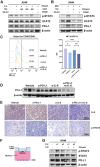
Methods: IL-6 levels were measured in MPE and paired serum samples from lung cancer patients, and correlations with PD-L1 expression and clinical outcomes were analyzed using publicly available datasets. RNA sequencing and immune deconvolution were used to assess immune cell infiltration. CAFs and immune cell infiltration were further evaluated using flow cytometry, immunohistochemistry, and multiplex immunofluorescence. In vitro co-culture systems were employed to simulate the MPE microenvironment and explore IL-6 interactions with CAFs, as well as its regulatory effect on tumor cell PD-L1 expression.
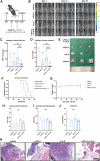
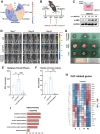
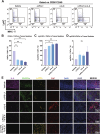
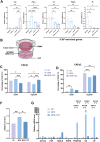
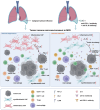
Results: IL-6 levels were significantly elevated in MPE compared to paired serum and correlated with higher PD-L1 expression and poor survival outcomes in lung cancer patients. In the MPE mouse model, combination therapy with IL-6 and PD-L1 blockade reduced MPE volume, tumor burden, and PD-L1 expression, while enhancing T cell infiltration and alleviating TME immunosuppression. IL-6 was found to drive a positive feedback loop with iCAFs, promoting an immunosuppressive environment. In vitro, IL-6 from the MPE upregulated tumor cell PD-L1 expression the IL-6/STAT3 pathway.
Conclusion: This study identifies IL-6 as a critical contributor of immune suppression and tumor progression in MPE. The combination of IL-6 and PD-L1 blockade effectively alleviated immunosuppression and reduced tumor burden, offering a potential therapeutic approach for MPE management.
Keywords: Cancer-associated fibroblast; IL-6; Immunotherapy; Lung cancer; Malignant pleural effusion; PD-L1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The studies using human specimens were approved by the Ethical Committee and Institutional Review Board of the Jinling Hospital (#2021DZGZR-YBB-063). The participants provided their informed consent to participate in the study. All animal experiments were approved by the Ethical Committee and Institutional Review Board of the Jinling Hospital (#2021DZGKJDWLS-0089). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion.Nat Nanotechnol. 2022 Feb;17(2):206-216. doi: 10.1038/s41565-021-01032-w. Epub 2021 Dec 16. Nat Nanotechnol. 2022. PMID: 34916656 Free PMC article.
-
Consistency Analysis of Programmed Death Ligand 1 Expression in Non-Small Cell Lung Cancer Between Pleural Effusion and Matched Primary Lung Cancer Tissues by Immunohistochemical Double Staining.Lab Invest. 2024 Jun;104(6):102058. doi: 10.1016/j.labinv.2024.102058. Epub 2024 Apr 16. Lab Invest. 2024. PMID: 38626874
-
Cytology cell blocks from malignant pleural effusion are good candidates for PD-L1 detection in advanced NSCLC compared with matched histology samples.BMC Cancer. 2020 Apr 22;20(1):344. doi: 10.1186/s12885-020-06851-z. BMC Cancer. 2020. PMID: 32321471 Free PMC article.
-
Therapeutic Implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade.Front Immunol. 2022 Jan 7;12:799455. doi: 10.3389/fimmu.2021.799455. eCollection 2021. Front Immunol. 2022. PMID: 35069581 Free PMC article. Review.
-
What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 3: PD-L1, Intracellular Signaling Pathways and Tumor Microenvironment.Int J Mol Sci. 2021 Nov 15;22(22):12330. doi: 10.3390/ijms222212330. Int J Mol Sci. 2021. PMID: 34830209 Free PMC article.
Cited by
-
Effect of intrapleural anti-Vascular Endothelial Growth Factor (VEGF) associated with nab paclitaxel in a murine model of malignant pleural effusion.BMC Cancer. 2025 Aug 2;25(1):1261. doi: 10.1186/s12885-025-14622-x. BMC Cancer. 2025. PMID: 40753384 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

