Bone marrow microenvironment in myelodysplastic neoplasms: insights into pathogenesis, biomarkers, and therapeutic targets
- PMID: 40349084
- PMCID: PMC12065391
- DOI: 10.1186/s12935-025-03793-z
Bone marrow microenvironment in myelodysplastic neoplasms: insights into pathogenesis, biomarkers, and therapeutic targets
Abstract
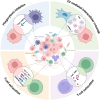
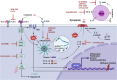
Myelodysplastic neoplasms (MDS) represent a heterogeneous group of malignant hematopoietic stem and progenitor cell (HSPC) disorders characterized by cytopenia, ineffective hematopoiesis, as well as the potential to progress to acute myeloid leukemia (AML). The pathogenesis of MDS is influenced by intrinsic factors, such as genetic insults, and extrinsic factors, including altered bone marrow microenvironment (BMM) composition and architecture. BMM is reprogrammed in MDS, initially to prevent the development of the disease but eventually to provide a survival advantage to dysplastic cells. Recently, inflammation or age-related inflammation in the bone marrow has been identified as a key pathogenic mechanism for MDS. Inflammatory signals trigger stress hematopoiesis, causing HSPCs to emerge from quiescence and resulting in MDS development. A better understanding of the role of the BMM in the pathogenesis of MDS has opened up new avenues for improving diagnosis, prognosis, and treatment of the disease. This article provides a comprehensive review of the current knowledge regarding the significance of the BMM to MDS pathophysiology and highlights recent advances in developing innovative therapies.
Keywords: Bone marrow microenvironment; Hematopoietic stem and progenitor cells; Ineffective hematopoiesis; Inflammation; Innovative therapies; Myelodysplastic neoplasms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

