The PancreasView gemcitabine transcriptomic signature predicts response to gemcitabine in patients with resected pancreatic ductal adenocarcinoma
- PMID: 40349133
- PMCID: PMC12065937
- DOI: 10.1093/oncolo/oyaf083
The PancreasView gemcitabine transcriptomic signature predicts response to gemcitabine in patients with resected pancreatic ductal adenocarcinoma
Abstract
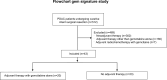
Introduction: In the current study, we aimed to assess the efficacy of a gemcitabine response predictive signature that is part of the PancreasView transcriptomic predictive tool (Gem + or Gem-).
Methods: We used a cohort of pancreatic ductal adenocarcinoma patients treated from the Massachusetts General Hospital who underwent upfront resection.
Results: In this cohort of 43 patients, 20 (46.5%) received adjuvant gemcitabine (GEM arm) and 23 (53.5%) did not receive any adjuvant chemotherapy. Among the 43 patients, the Gem signature defined a subgroup of 16 patients (37.2%) who were sensitive (Gem+) and 27 (62.8%), who were resistant to gemcitabine (Gem-). The Gem+ patients who received adjuvant gemcitabine had significantly better median disease-free survival (DFS) compared to the Gem- patients (NR until 72 months of follow-up vs 19.0 months; stratified hazard ratio [HR]: 0.19; 95% CI, 0.04-0.86; P = .032) and longer median cancer-specific survival (CSS) (NR until 96 months of follow-up vs 37.0 months; stratified HR: 0.18; 95% CI, 0.04-0.85; P = .030) when treated with gemcitabine. The gemcitabine signature remained an independent predictive factor for DFS (HR: 0.41; 95% CI, 0.19-0.89; P = .024) and CSS (HR: 0.47; 95% CI, 0.22-1.23; P = .059) after adjusting for clinicopathological characteristics in an unstratified univariate Cox hazard model.
Conclusions: This validation of the gemcitabine predictive transcriptomic signature in an independent cohort from Massachusetts General Hospital reinforces the robustness and reliability of this tool. This study highlights the potential of the signature to aid in the personalization of chemotherapy and enhance patient outcomes in pancreatic ductal adenocarcinoma.
Keywords: PancreasView; PDAC; clinical trial; personalized treatment; transcriptomic signatures.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
N.J.D. and J.L.I. are co-founders of Predicting Med. All other authors have declared no conflicts of interest.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al.Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. https://doi.org/10.3322/caac.21660 - DOI - PubMed
-
- van der Sijde F, van Dam JL, Groot Koerkamp B, et al.Treatment response and conditional survival in advanced pancreatic cancer patients treated with FOLFIRINOX: a multicenter cohort study. J Oncol. 2022;2022:8549487. https://doi.org/10.1155/2022/8549487 - DOI - PMC - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al.FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. https://doi.org/10.1056/nejmoa1011923 - DOI - PubMed
-
- Conroy T, Hammel P, Hebbar M, et al.; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395-2406. https://doi.org/10.1056/NEJMoa1809775 - DOI - PubMed
-
- Fraunhoffer N, Hammel P, Conroy T, et al.Development and validation of AI-assisted transcriptomic signatures to personalize adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Ann Oncol. 2024;S0923-7534:00741-00745. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

