Evaluation of the effectiveness and safety of avapritinib in real-world Spanish cases with gastrointestinal stromal tumor and D842V-PDGFRA mutation
- PMID: 40349140
- PMCID: PMC12065942
- DOI: 10.1093/oncolo/oyaf062
Evaluation of the effectiveness and safety of avapritinib in real-world Spanish cases with gastrointestinal stromal tumor and D842V-PDGFRA mutation
Abstract
Introduction: Gastrointestinal stromal tumors (GISTs) are the most common sarcoma subtype. Patients with unresectable or metastatic GISTs harboring the D842V mutation in the PDGFRA gene have a poor prognosis due to intrinsic resistance to imatinib and all other approved tyrosine kinase inhibitors. Avapritinib, targeting this mutation, is the first agent approved for patients with unresectable or metastatic GIST that have the PDGFRA D842V mutation. This study assesses the effectiveness and safety of avapritinib in real-world clinical scenarios involving Spanish patients with this mutation.
Materials and methods: The AVARWE study is a descriptive, retrospective, multicenter observational study of 21 patients treated with avapritinib across 13 Spanish centers from June 9, 2023, to December 18, 2023. Data collected included patient demographics, disease characteristics, treatment history, and response rates based on RECIST criteria. The main outcomes, progression-free survival (PFS) and overall survival (OS), were measured, with safety assessed through adverse events documentation according to CTCAE criteria.
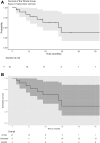
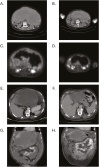
Results: Median PFS 35.6 was months and median OS was 42.2 months, with survival rates at 1, 5, and 3 years demonstrating avapritinib effectiveness. The objective response rate was 76.2% for partial response and 4.8% for complete response. Avapritinib enabled surgical intervention in previously unresectable cases and was generally well-tolerated, with manageable adverse events.
Conclusion: Avapritinib extends PFS and OS among patients with PDGFRA D842V-mutant GIST in real-world practice, mirroring pivotal trial outcomes. Its substantial activity supports its use as a first-line therapy for this subgroup. The manageable safety profile reinforces avapritinib viability for routine use. Given the rarity of these cases, it is advised to consult sarcoma-expert units.
Keywords: PDGFRA; D842V mutation; GIST; avapritinib; gastrointestinal stromal tumor; outcomes; treatment.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
I.N.H.: declares no conflict of interest. C.G.P.: declares no conflict of interest. J.R.D.: declares no conflict of interest. A.N.: has served as a consultant and received speaking fees at advisory boards for Seattle Genetics, received a research grant from Eisai, and received support for attending meetings and/or travel for Lilly, MSD, Novartis, and Roche outside the submitted work. M.A.S.G. has served as a consultant and received speaking fees at educational events for Takeda, Roche, and Pfizer and received support for attending meetings and/or travel for Roche and PharmaMar. A.G.A.: declares no conflict of interest. H.A: declares no conflict of interest. P.A.M.: has served as a consultant and received speaking fees for BMS, MSD, Novartis, Pierre-Fabre, and Sanofi. E.C.Q.: has served as a consultant and received speaking fees for Bristol Myers, Novartis, PharmaMar, and ROVI. R.D.B.: declares no conflict of interest. J.M.G.: has served as a consultant and received speaking fees at advisory boards for Astra-Zeneca, Clovis, GSK-Thesaro, PharmaMar, received a research grant from GSK, Roche and received support for attending meetings and/or travel from GSK-Thesaro, MSD, and PharmaMar. G.M.: reports receipt of a fee for participation in advisory boards from Deciphera Pharmaceuticals, PharmaMar, Clovis oncology, GSK/Tesaro, Lilly, Pfizer, Boehringer Ingelheim, EISAI; receipt of a fee as an invited speaker from Roche, AstraZeneca, GSK/Tesaro, Clovis Oncology, Medicamenta, EISAI, PharmaMar, Bayer, Lilly, IPSEN, MSD; receipt of travel grants from Roche, PharmaMar, GSK/Tesaro, MSD, Medicamenta, Pfizer, Angelini, Novartis, Merck, Lilly, EISAI. J.M.G.: has served as a consultant and received speaking fees at advisory boards for Astra-Zeneca, Clovis, GSK-Thesaro, PharmaMar, received a research grant from GSK, Roche and received support for attending meetings and/or travel from GSK-Thesaro, MSD, and PharmaMar. V.V.P.: has served as a consultant and received speaking fees at advisory boards for Pfizer, and received institutional funding for trials and research from Boehringer Ingelheim, BioNTech, Regeneron, Ipsen Pharma, and received support for attending meetings and/or travel for Pfizer. GSK, Roche, and received support for attending meetings and/or travel from GSK-Thesaro, MSD, and PharmaMar. J.V.R.: has received lecture fees from MSD and travel and education funding from MSD, Astra Zeneca, Advanz pharma, and AAA. C.S. reports receipt of a fee for participation in advisory boards from Blueprint Medicines, Cogent Biosciences, Deciphera Pharmaceuticals, IDRx, Immunicum, New Bay; receipt of a fee as an invited speaker from Roche, PharmaMar; receipt of research grants to institution from IDRx; receipt of travel grants from Bayer AG, Gilead, Pfizer, PharmaMar.
Figures


References
-
- Ducimetière F, Lurkin A, Ranchère-Vince D, et al.Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. https://doi.org/10.1371/journal.pone.0020294 - DOI - PMC - PubMed
-
- Schaefer IM, DeMatteo RP, Serrano C.. The GIST of advances in treatment of advanced gastrointestinal stromal tumor. Am Soc Clin Oncol Educ Book. 2022;42:1-15. https://doi.org/10.1200/EDBK_351231 - DOI - PMC - PubMed
-
- Serrano C, Martín-Broto J, Asencio-Pascual JM, et al.2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. 2023;15:17588359231192388. https://doi.org/10.1177/17588359231192388 - DOI - PMC - PubMed
-
- Casali PG, Blay JY, Abecassis N, et al.; ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20-33. https://doi.org/10.1016/j.annonc.2021.09.005 - DOI - PubMed
-
- Klug LR, Khosroyani HM, Kent JD, Heinrich MC.. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol. 2022;19:328-341. https://doi.org/10.1038/s41571-022-00606-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

