Correlation between tumor growth rate and survival in patients with metastatic breast cancer treated with trastuzumab deruxtecan
- PMID: 40349141
- PMCID: PMC12065935
- DOI: 10.1093/oncolo/oyaf057
Correlation between tumor growth rate and survival in patients with metastatic breast cancer treated with trastuzumab deruxtecan
Abstract
Background: Previous studies in multiple metastatic tumors treated with diverse anticancer agents including immunotherapy, chemotherapy, mAb, and TKIs have suggested the rate of tumor growth (g-score) is inversely associated with survival.
Methods: We performed a retrospective analysis of patients with metastatic breast cancer (mBC) treated with trastuzumab deruxtecan (T-DXd), ado-trastuzumab emtansine (T-DM1), or chemotherapy to investigate the impact of those therapies on g-score and explore the association of g-score with clinical outcomes. This is the first report assessing g-score in tumors treated with an ADC.
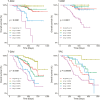
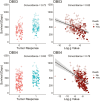
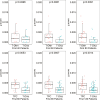
Results: We investigated the association of g-score with progression-free (PFS) and overall survival (OS) in 2 phase 3 studies in patients with HER2 + mBC (DESTINY-Breast03 (DB-03)) and HER2-low mBC (DESTINY-Breast04 (DB-04)). After grouping patients according to quartiles of g-scores, we explored the association between g-score and PFS/OS using Kaplan-Meier plots and Cox regression models. The median g-score was higher for T-DM1, suggesting a faster growth rate at 0.0009/day vs that for T-DXd at 0.0002/day (P < .0001). Additionally, with data collection stopped at the time of database lock, 23% and 48% of tumors demonstrated only regression without growth in the T-DM1 and T-DXd arms, respectively. In DB-04, median g was 0.0018/day and 0.0006/day (P < .0001); with 17% and 32% of tumors demonstrating only regression with treatment of physician's choice (TPC) and T-DXd, respectively.
Conclusions: Compared to T-DM1 and TPC therapies, T-DXd significantly reduced the rate of tumor growth in the overall population and across subgroups. In both studies, the tumor growth rate was inversely associated with PFS and OS. In addition, it showed improved concordance with survival compared to ORR. The use of tumor growth rate as an intermediate endpoint may potentially accelerate drug development and reduce a patient's exposure to agents with limited or no activity.
Keywords: biomarkers; intermediate endpoint; metastatic breast cancer; tumor growth rate.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
P.H.: Stock and employment: Daiichi Sankyo. D.G.: Stock and employment: Daiichi Sankyo. H.Z.: Stock and employment: Daiichi Sankyo. X.M.: Stock and employment: Daiichi Sankyo. Y.E.: Stock and employment: Daiichi Sankyo. A.L.: Stock and employment: Daiichi Sankyo. D.L.: Stock and employment: Daiichi Sankyo. S.B.: Dr. Bates received an honorarium for a research symposium at Daiichi-Sankyo; received research funding from Pfizer and RenovoRx; and participates in the scientific advisory boards of Elmedex, Acrivon, and Pegascy. A.TF.: No COI. O.R.: Stock and employment: Daiichi Sankyo.
Figures


References
-
- Stewart DJ, Stewart AA, Wheatley-Price P, et al.The importance of greater speed in drug development for advanced malignancies. Cancer Med. 2018;7:1824-1836. https://doi.org/10.1002/cam4.1454 - DOI - PMC - PubMed
-
- Alexander BM, Cloughesy TF.. Adult glioblastoma. J. Clin. Oncol. 2017;35:2402-2409. https://doi.org/10.1200/JCO.2017.73.0119 - DOI - PubMed
-
- Kesselheim AS, Hwang TJ, Franklin JM.. Two decades of new drug development for central nervous system disorders. Nat Rev Drug Discovery. 2015;14:815-816. https://doi.org/10.1038/nrd4793 - DOI - PubMed
-
- Yeh C, Zhou M, Sigel K, et al.Tumor growth rate informs treatment efficacy in metastatic pancreatic adenocarcinoma: application of a growth and regression model to pivotal trial and real‐world data. Oncologist. 2022;28:139-148. https://doi.org/10.1093/oncolo/oyac217 - DOI - PMC - PubMed
-
- U.S. Food and Drug Administration. Clinical Trial Endpoints for the Approval of Non Small Cell Lung Cancer Drugs and Biologics Guidance for Industry. 2015. https://www.fda.gov/media/116860/download
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

