Microglial MS4A4A Protects against Epileptic Seizures in Alzheimer's Disease
- PMID: 40349168
- PMCID: PMC12165070
- DOI: 10.1002/advs.202417733
Microglial MS4A4A Protects against Epileptic Seizures in Alzheimer's Disease
Abstract
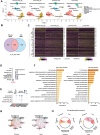
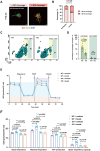
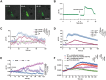
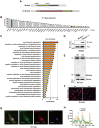
Alzheimer's disease (AD) is a predominant neurodegenerative disorder worldwide, with epileptic seizures being a common comorbidity that can exacerbate cognitive deterioration in affected individuals, thus highlighting the importance of early therapeutic intervention. It is determined that deletion of Ms4a4a, an AD-associated gene, exacerbates seizures in amyloid β (Aβ)-driven AD mouse model. MS4A4A is significantly upregulated in brain lesions in patients with epilepsy. Single-cell sequencing reveals that MS4A4A is highly expressed in microglia within these lesions, linked to enhanced phagocytic activity. Mechanistic investigation delineates that deletion of Ms4a4a impairs microglial phagocytosis, accompanied by diminished calcium influx and disruptions in mitochondrial metabolic fitness. The cytosolic fragment of Ms4a4a is anchored to the cytoskeletal components, supporting its critical role in mediating phagocytosis. Induction of Ms4a4a through central delivery of LNP-Il4 alleviates seizure conditions. Collectively, these findings identify Ms4a4a as a potential therapeutic target for managing seizures in AD treatment.
Keywords: Alzheimer's disease; MS4A4A; epilepsy; microglia; phagocytosis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pease M., Gupta K., Moshé S. L., Correa D. J., Galanopoulou A. S., Okonkwo D. O., Gonzalez‐Martinez J., Shutter L., Diaz‐Arrastia R., Castellano J. F., Nat. Rev. Neurol. 2024, 20, 298. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
