Sex- and age-dependent neurovascular abnormalities linked to neuroinflammation lead to exacerbated post-ischemic brain injury in Marfan syndrome mice
- PMID: 40349485
- PMCID: PMC12139021
- DOI: 10.1016/j.redox.2025.103662
Sex- and age-dependent neurovascular abnormalities linked to neuroinflammation lead to exacerbated post-ischemic brain injury in Marfan syndrome mice
Abstract
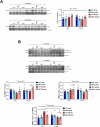
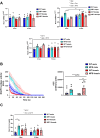
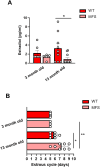
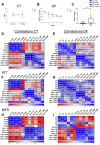
Fibrillin 1 gene (Fbn1) mutations cause Marfan syndrome (MFS), triggering life-threatening aortic complications and multi-organ effects. MFS is increasingly linked to neurovascular complications, amplified by aortic surgery risks. However, the impact of MFS on the brain remains unclear, including the roles of sex, aging, and their contribution to cerebral injury. This study examines brain alterations and their role in cerebral ischemic injury in an MFS mouse model. RNA-seq analysis of young (3-month-old) and aged (13-month-old) male and female wild-type and MFS (Fbn1C1041G/+) mice revealed disruptions in TGF-β and extracellular matrix (ECM) pathways in MFS brains, most pronounced in young males and aged females with reduced estrogen levels. Inflammatory pathways were upregulated across all MFS mice. Consequently, changes in TGF-β signaling, ECM turnover, redox stress and inflammatory pathways were assessed through RT-qPCR, immunostaining, Western blot, lucigenin chemiluminescence, spectrophotometry, HPLC, and synchrotron radiation-based microspectroscopy, while cerebrovascular properties were assessed by pressure myography and confocal microscopy in the basilar artery. Aged MFS mice showed decreased brain TGF-β1 levels, while dysregulated collagen turnover was only observed in female MFS mice. Despite increased NADPH oxidase activity and redox damage in the corpus callosum of male MFS mice, brain redox stress levels remain largely unchanged. Young female MFS mice exhibited hypertrophic remodeling of the basilar artery. Remarkably, neuroinflammation driven by reactive gliosis increased in MFS mice, regardless of sex and age. To determine the impact on ischemic vulnerability, young mice underwent bilateral common carotid artery occlusion (5 min)/reperfusion (3 days). MFS mice showed greater post-ischemic brain damage, evidenced by worsened behavioral impairments, hippocampal neurodegeneration, and neuroinflammation. This study identifies sex- and age-dependent disruptions in TGF-β1, ECM, and cerebrovascular integrity in MFS mice. Persistent neuroinflammation and increased vulnerability to post-ischemic brain injury suggests that MFS patients, alongside well-documented aortic complications, have an intrinsic predisposition to cerebral damage.
Keywords: Extracellular matrix; Ischemic brain injury; Marfan syndrome; Neuroinflammation; Neurovascular complications; fibrillin 1.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Figures







References
-
- Writing Committee Members, Isselbacher E.M., Preventza O., Hamilton Black J., 3rd, Augoustides J.G., Beck A.W., Bolen M.A., Braverman A.C., Bray B.E., Brown-Zimmerman M.M., Chen E.P., Collins T.J., DeAnda A., Jr., Fanola C.L., Girardi L.N., Hicks C.W., Hui D.S., Schuyler Jones W., Kalahasti V., Kim K.M., Milewicz D.M., Oderich G.S., Ogbechie L., Promes S.B., Ross E.G., Schermerhorn M.L., Singleton Times S., Tseng E.E., Wang G.J., Woo Y.J., Peer Review Committee Members, Faxon D.P., Upchurch G.R., Jr., Aday A.W., Azizzadeh A., Boisen M., Hawkins B., Kramer C.M., Luc J.G.Y., MacGillivray T.E., Malaisrie S.C., Osteen K., Patel H.J., Patel P.J., Popescu W.M., Rodriguez E., Sorber R., Tsao P.S., Santos Volgman A., Aha/Acc Joint Committee Members, Beckman J.A., Otto C.M., O'Gara P.T., Armbruster A., Birtcher K.K., de Las Fuentes L., Deswal A., Dixon D.L., Gorenek B., Haynes N., Hernandez A.F., Joglar J.A., Jones W.S., Mark D., Mukherjee D., Palaniappan L., Piano M.R., Rab T., Spatz E.S., Tamis-Holland J.E., Woo Y.J. ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. J. Thorac. Cardiovasc. Surg. 2022;166(5):e182–e331. doi: 10.1016/j.jtcvs.2023.04.023. 2023. - DOI - PMC - PubMed
-
- Domingo R.A., Perez-Vega C., Tripathi S., Santos J.M., Ramos-Fresnedo A., Erben Y.M., Freeman W.D., Sandhu S.S., Huynh T., Williams L., Bendok B.R., Brinjikji W., Tawk R.G., Fox W.C. Intracranial aneurysms in patients with marfan syndrome: a multicenter propensity-matched analysis. World Neurosurg. 2021;155:e345–e352. doi: 10.1016/j.wneu.2021.08.065. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

