Upadacitinib as monotherapy in patients with rheumatoid arthritis and prior inadequate response to methotrexate: results at 260 weeks from the SELECT-MONOTHERAPY randomised study
- PMID: 40350200
- PMCID: PMC12067782
- DOI: 10.1136/rmdopen-2024-005051
Upadacitinib as monotherapy in patients with rheumatoid arthritis and prior inadequate response to methotrexate: results at 260 weeks from the SELECT-MONOTHERAPY randomised study
Abstract
Introduction: The phase III SELECT-MONOTHERAPY trial (NCT02706951) demonstrated the safety and efficacy of upadacitinib (UPA) monotherapy through 84 weeks in patients with rheumatoid arthritis who responded inadequately to methotrexate. Here we report week 260 results.
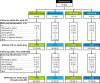
Methods: Patients were randomised to continue methotrexate or UPA 15 mg (UPA15) or 30 mg (UPA30) monotherapy for 14 weeks. From week 14, patients continuing methotrexate switched to UPA15 or UPA30 per prespecified assignment; patients randomised to UPA continued treatment. Following a protocol amendment, all cohorts switched to open-label UPA15. Safety was summarised using exposure-adjusted event and incidence rates. Efficacy was reported as observed and using non-responder imputation (NRI).
Results: Of 648 randomised patients, 598 entered the long-term extension. Of these, 249 (41.6%) discontinued study drug by week 260 primarily due to adverse events (14.5%), consent withdrawal (9.9%), lost to follow-up (3.3%), lack of efficacy (2.2%), COVID-19 (0.7%) or other reasons (11.0%). Rates of herpes zoster, non-melanoma skin cancer, hepatic disorder, neutropenia, lymphopenia and creatine kinase elevation were higher with UPA30 versus UPA15. Long-term UPA safety data were consistent with the established UPA safety profile. Based on NRI, >39% of patients treated continuously with UPA achieved low disease activity per Clinical Disease Activity Index ≤10 (UPA15, n=93/217; UPA30, n=91/215) and 28-joint Disease Activity Score using C reactive protein ≤3.2 (UPA15, n=90/217; UPA30, n=94/215) at week 260. Efficacy was similar among patients switching from methotrexate to UPA.
Conclusion: No new safety risks were identified with long-term UPA treatment. UPA monotherapy was efficacious in treating rheumatoid arthritis through week 260.
Trial registration number: NCT02706951.
Keywords: Arthritis, Rheumatoid; Methotrexate; Pain; Patient Reported Outcome Measures; Therapeutics.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JSS has received research grants, consulting fees and/or personal fees from AbbVie, Amgen, AstraZeneca, Astro, BMS, Celgene, Celltrion, Chugai, Eli Lilly, Gilead, ILTOO, Janssen, Merck Sharp & Dohme, Novartis, Novartis-Sandoz, Pfizer, Roche, Samsung and UCB. PE has received research grants, consulting fees and/or honoraria from AbbVie, BMS, Eli Lilly, Gilead, Merck Sharp & Dohme, Novartis, Pfizer, Roche and Samsung. WR has received consulting fees from AbbVie, BMS, Genentech and Pfizer. YT has received speaking fees and/or honoraria from AbbVie, Asahi-Kasei, Astellas, AstraZeneca, BI, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Pfizer, Taisho and UCB, and has received research grants from BI, Chugai and Taisho. JIV has received honoraria from AbbVie. MJ has received research grants, consulting fees, speaking fees and/or honoraria from AbbVie, Amgen, Celgene, Eli Lilly, Medac, Novartis, Pfizer and Takeda. KK, KMC, NK, CP and SM are full-time employees of AbbVie and may hold AbbVie stock and/or stock options. SBC has received research grants and/or consulting fees from AbbVie, Amgen, BI, Eli Lilly, Gilead, Pfizer, Roche and Sandoz.
Figures





References
-
- Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372:375–82. doi: 10.1016/S0140-6736(08)61000-4. - DOI - PubMed
-
- van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YPM, et al. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis. 2007;66:1356–62. doi: 10.1136/ard.2006.066662. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
