Luminescent mouse model of endometriosis: three-dimensional morphology of lesions and cytokine profiles
- PMID: 40350305
- PMCID: PMC12665997
- DOI: 10.1538/expanim.25-0044
Luminescent mouse model of endometriosis: three-dimensional morphology of lesions and cytokine profiles
Abstract
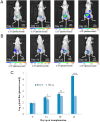
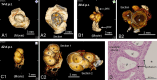
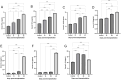
The pathophysiology of endometriosis remains incompletely understood, necessitating the development of effective animal models for research. We generated and characterized a luminescent endometriosis mouse model utilizing luminescent B6-CAG-ELuc transgenic mice as uterine tissue donors and B6.Cg-c/c-hr/hr mice as recipients, enabling non-invasive in vivo imaging. Following transplantation of minced uterine tissue fragments into the peritoneal cavity of recipients, we monitored lesion growth via in vivo imaging system on 0, 14, 28, 42 days post transplantation. Morphology of the lesion was observed by dissecting microscopy, X-ray micro-computed tomography, and conventional histology. Inflammation-related serum cytokines were quantified using multiplex immunobeads assay. The growth of endometriotic lesions was efficiently observed by bioluminescence from day 0 through 42 days post transplantation. Comprehensive morphological observations revealed typical endometriotic lesions consisted of multiple fluid-filled cysts lined with single-layered epithelium, associated with glandular epithelial tissues and interstitial stroma. The level of IL-1β, IL-2, IL-6, IL-10, IL-12p70, IFN-γ, and TNF-α was quantified simultaneously in each serum sample to evaluate the temporal changes of each cytokine, showing four distinct patterns: IFN-γ and TNF-α showed continuous increase, IL-12p70 and IL-1β demonstrated gradual increase followed by marked elevation, IL-6 and IL-2 exhibited dramatic increase in later stages, while IL-10 showed transient increase followed by gradual decrease. In conclusion, this luminescent endometriosis mouse model using B6 luminescent transgenic mice as uterine tissue donor and B6.Cg-c/c-hr/hr recipient could be used to investigate comprehensive cytokine profiling in the development of endometriosis.
Keywords: bioluminescence; cytokine; endometriosis; in vivo imaging; micro-CT.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

