Denosumab as an immune modulator in HER2-negative early breast cancer: results of the window-of-opportunity D-BIOMARK clinical trial
- PMID: 40350430
- PMCID: PMC12067755
- DOI: 10.1186/s13058-025-01996-w
Denosumab as an immune modulator in HER2-negative early breast cancer: results of the window-of-opportunity D-BIOMARK clinical trial
Abstract
Background: The RANK pathway has been extensively investigated for its role in bone resorption; however, its significance extends beyond bone metabolism. Preclinical models suggest that inhibition of RANK signaling can prevent mammary tumor development by reducing proliferation and tumor cell survival. Additionally, both preclinical and clinical data support the ability of RANK pathway inhibitors to enhance the anti-tumor immune response.
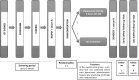
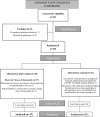
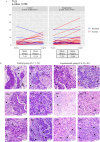
Methods: D-BIOMARK is a prospective, randomized window-of-opportunity clinical trial assessing the biological effects of denosumab, a monoclonal antibody against RANKL, in patients with HER2-negative early breast cancer. The study aims to assess denosumab's impact on breast tumor cell proliferation, apoptosis, and its potential to influence the tumor immune microenvironment. A total of 60 patients were enrolled and randomized 2:1 to receive two doses of single agent denosumab (120 mg one week apart) before surgery or to the control arm (no treatment). Fifty-eight patients were evaluated, 27 pre-menopausal and 31 post-menopausal women, 48 with luminal tumors and 10 with triple negative breast cancer. Paired tumor samples were collected to compare baseline (core biopsy) and surgical (surgical specimen) time points, as well as serum samples at both time points.
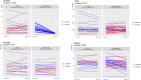
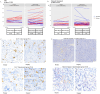
Results: Denosumab demonstrated its ability to reduce serum free RANKL levels (experimental p < 0.001, control p = 0.270). However, a reduction in tumor cell proliferation or cell survival was not observed. A denosumab-driven increase in tumor infiltrating lymphocytes (TILs) was observed (experimental p = 0.001, control p = 0.060), particularly in the luminal B-like population (experimental p = 0.012, control p = 0.070) and a similar trend in the TNBC group (experimental p = 0.079, control p = 0.237). Denosumab led to increased TILs in both pre-menopausal (experimental p = 0.048, control p = 0.639) and post-menopausal (experimental p = 0.041, control p = 0.062) women with luminal tumors. RANK protein expression in tumor and stroma was associated with markers of tumor aggressiveness but an increase in TILs was observed in the experimental arm, irrespectively of RANK and RANKL expression in tumor or stromal cells.
Conclusions: The D-BIOMARK trial suggests a potential role for denosumab as an immune-enhancing agent in early HER2-negative breast cancer. Although preoperative denosumab did not reduce tumor proliferation or increased apoptosis, it led to an increase in TILs, particularly in luminal B-like tumors. These findings underscore the importance of further investigation into the multifaceted aspects of the RANK pathway. Trial registration EudraCT number: 2016-002678-11 registered on June 15, 2018.
Clinicaltrials: gov identifier: NCT03691311, retrospectively registered on September 04, 2018.
Keywords: Breast cancer; Denosumab; HER2-negative; Immune enhancer; RANK; RANKL; TILs; Tumor cell proliferation; Tumor cell survival.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Informed consent: Informed consent was obtained from all subjects involved in the study. Institutional review board statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the “Institut Català d’Oncologia de L’Hospitalet (ICO-L’Hospitalet)” (protocol code PR035/21) Competing interests: The authors declare no competing interests.
Figures

References
-
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–9. - PubMed
-
- Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–7. - PubMed
-
- González-Suárez E, Sanz-Moreno A. RANK as a therapeutic target in cancer. FEBS J. 2016;283(11):2018–33. 10.1111/febs.13645. - PubMed
-
- Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(12):1719–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

