Digoxin promotes anoikis of circulating cancer cells by targeting Na+/K+-ATPase α3-isoform
- PMID: 40350473
- PMCID: PMC12066707
- DOI: 10.1038/s41419-025-07703-z
Digoxin promotes anoikis of circulating cancer cells by targeting Na+/K+-ATPase α3-isoform
Abstract
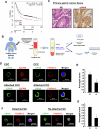
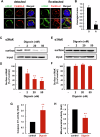
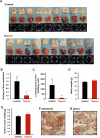
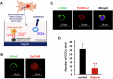
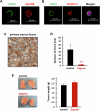
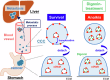
Circulating cancer cells (CCCs) are closely related to the process of distant metastasis. In early step of the metastasis cascade, CCCs must evade the detachment-induced cell death (anoikis) for their survival. Here, we examined whether Na+/K+-ATPase α3-isoform (α3NaK) in CCCs contributes to avoidance of anoikis. In CCCs isolated from gastric cancer patients, α3NaK was predominantly localized in the plasma membrane (PM), but it moved to the cytoplasm when the CCCs were attached to culture dishes. The CCCs showed significant expression of integrin α5 but not fibronectin, one of components of the extracellular matrix (ECM). In human gastric cancer MKN45 cells, digoxin (20 and 50 nM), a cardiac glycoside, significantly inhibited the enzyme activity and translocation (from cytoplasm to PM) of α3NaK, while they had no significant effect on ubiquitous Na+/K+-ATPase α1-isoform (α1NaK) in the PM. The translocation of α3NaK required the loss of ECM components from the cells. Additionally, digoxin significantly enhanced caspase 3/7 activity, as well as the expression of cleaved caspase 3, while reducing the viability of detached (floating) cells. In the MKN45 xenograft mouse model, intraperitoneal administration of digoxin (2 mg/kg/day) significantly decreased the number of CCCs and suppressed their liver metastasis. Our results suggest that α3NaK plays an essential role in the survival of CCCs in gastric cancer, and that digoxin enhances anoikis in detached (metastatic) gastric cancer cells by inhibiting the α3NaK translocation from cytoplasm to PM, thereby reducing CCCs. Targeting α3NaK may be a promising therapeutic strategy against CCC survival.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Montagnani F, Crivelli F, Aprile G, Vivaldi C, Pecora I, De Vivo R, et al. Long-term survival after liver metastasectomy in gastric cancer: systematic review and meta-analysis of prognostic factors. Cancer Treat Rev. 2018;69:11–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

