Solid-Liquid Interfacial Free Energy from Computer Simulations: Challenges and Recent Advances
- PMID: 40350612
- PMCID: PMC12123632
- DOI: 10.1021/acs.chemrev.4c00833
Solid-Liquid Interfacial Free Energy from Computer Simulations: Challenges and Recent Advances
Abstract
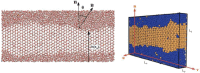
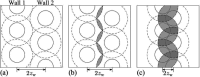
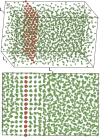
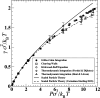
The study of interfacial properties in liquid-liquid and liquid-vapor systems has a history of nearly 200 years, with significant contributions from scientific luminaries such as Thomas Young and Willard Gibbs. However, a similar level of understanding of solid-liquid interfaces has emerged only more recently, largely because of the numerous complications associated with the thermodynamics needed to describe them. The accurate calculation of the interfacial free energy of solid-liquid systems is central to determining which interfaces will be observed and their properties. However, designing and analyzing the molecular dynamics simulations required to do this remains challenging, unlike the liquid-liquid or liquid-vapor cases, because of the unique complications associated with solid-liquid systems. Specifically, the lattice structure of solids introduces spatial directionality, and atomic configurations in solids can be altered by stretching. The primary aim of this review is to provide an overview of the numerical approaches developed to address the challenge of calculating the interfacial free energy in solid-liquid systems. These approaches are classified as (i) direct methods, which compute interfacial free energies explicitly, albeit often through convoluted procedures, and (ii) indirect methods, which derive these free energies as secondary results obtained from the analysis of simulations of an idealized experimental configuration. We also discuss two key topics related to the calculation of the interfacial free energy of solid-liquid systems: nucleation theory and curved interfaces, which represent important problems where research remains highly active.
Figures






References
-
- Brantley, S. L. ; White, A. F. ; Hodson, M. E. . Surface area of primary silicate minerals. In Growth, dissolution and pattern formation in geosystems; Springer, 1999; 291–326.
-
- Hoose C., Möhler O.. Heterogeneous ice nucleation on atmospheric aerosols: a review of results from laboratory experiments. Atmospheric Chemistry and Physics. 2012;12(20):9817–9854. doi: 10.5194/acp-12-9817-2012. - DOI
-
- Kalikmanov, V. I. Classical nucleation theory. In Nucleation theory; Springer, 2013; pp 17–41.
Publication types
LinkOut - more resources
Full Text Sources

