A Photonastic Prototissue Capable of Photo-Mechano-Chemical Transduction
- PMID: 40350988
- PMCID: PMC12510286
- DOI: 10.1002/adma.202502830
A Photonastic Prototissue Capable of Photo-Mechano-Chemical Transduction
Abstract
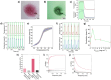
Despite recent significant advances in the controlled assembly of protocell units into complex 3D architectures, the development of prototissues capable of mimicking the sophisticated energy transduction processes fundamental to living tissues remains a critical unmet challenge in bottom-up synthetic biology. Here a synthetic approach is described to start addressing this challenge and report the bottom-up chemical construction of a photonastic prototissue endowed with photo-mechano-chemical transduction capabilities. For this, novel protocells enclosing photothermal transducing proto-organelles based on gold nanoparticles and a thermoresponsive polymeric proto-cortex are developed. These advanced protocell units are assembled into prototissues capable of light-induced reversible contractions and complex motions, which can be exploited to reversibly switch off a coordinated internalized enzyme metabolism by blocking the access of small substrate molecules. Overall, the work provides a synthetic pathway to constructing prototissues with sophisticated energy transduction mechanisms, enabling the rational design of emergent behaviors in synthetic materials and addressing critical challenges in bottom-up synthetic biology and bioinspired materials engineering.
Keywords: energy transduction; far‐from‐equilibrium material; photonastic behaviour; protocell; prototissue.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Needleman D., Dogic Z., Nat. Rev. Mater. 2017, 2, 17048.
-
- Livshits M. Y., Razgoniaev A. O., Arbulu R. C., Shin J., McCullough B. J., Qin Y., Ostrowski A. D., Rack J. J., ACS Appl. Mater. Interfaces 2018, 10, 37470. - PubMed
-
- Alcinesio A., Cazimoglu I., Kimmerly G. R., Restrepo Schild V., Krishna Kumar R., Bayley H., Adv. Funct. Mater. 2021, 32, 2107773.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

