Effects of Interfacial Adhesion on Lithium Plating Location in Solid-State Batteries with Carbon Interlayers
- PMID: 40351107
- PMCID: PMC12288772
- DOI: 10.1002/adma.202502114
Effects of Interfacial Adhesion on Lithium Plating Location in Solid-State Batteries with Carbon Interlayers
Abstract
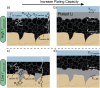
Carbon interlayers have been implemented in "anode-free" solid-state batteries to improve the uniformity and reversibility of lithium deposition by controlling the location of Li plating. However, there remains a lack of fundamental understanding of the detailed role of how these interlayers function during in situ Li formation. In this study, the relationships between the interfacial adhesion of the carbon interlayer to the solid electrolyte and the location of Li plating are investigated. By varying the lamination pressure used during manufacturing, the ability to systematically tune the resulting interfacial adhesion is demonstrated. Mechanical peel tests are performed, and a 4-fold increase in interfacial toughness is measured as the lamination pressure increases from 100 to 400 MPa. Post-mortem electron microscopy revealed that the location of Li plating with respect to the carbon interlayer transitions from the interface with the solid electrolyte to the current collector above a threshold interfacial toughness, which is consistent when the interlayer material is changed from amorphous to hard carbon. These findings highlight the role of electro-chemo-mechanical relationships in systematically controlling Li deposition in solid-state batteries when interlayers are present.
Keywords: adhesion; anode‐free; carbon interlayer; lithium metal anode; mechanical properties; solid‐state battery.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sandoval S. E., Haslam C. G., Vishnugopi B. S., Liao D. W., Yoon J. S., Park S. H., Wang Y., Mitlin D., Hatzell K. B., Siegel D. J., Mukherjee P. P., Dasgupta N. P., Sakamoto J., McDowell M. T., Nat. Mater. 2025, 24, 673. - PubMed
-
- Tian Y., An Y., Wei C., Jiang H., Xiong S., Feng J., Qian Y., Nano Energy 2020, 78, 105344.
-
- Krauskopf T., Dippel R., Hartmann H., Peppler K., Mogwitz B., Richter F. H., Zeier W. G., Janek J., Joule 2019, 3, 2030.
-
- Davis A. L., Kazyak E., Liao D. W., Wood K. N., Dasgupta N. P., J. Electrochem. Soc. 2021, 168, 070557.
Grants and funding
LinkOut - more resources
Full Text Sources

