Vesiculation as potential novel pathogenic mechanism in autoimmune hemolytic anemia
- PMID: 40351186
- PMCID: PMC12168431
- DOI: 10.1111/trf.18270
Vesiculation as potential novel pathogenic mechanism in autoimmune hemolytic anemia
Abstract
Background: Autoimmune hemolytic anemia (AIHA) is typically mediated by immunoglobulin G (IgG) or immunoglobulin M (IgM) antibodies, and more rarely by immunoglobulin A (IgA). The mechanism of red blood cell (RBC) destruction in IgA-mediated AIHA is not well understood. We report a case of severe AIHA with intravascular hemolysis, positive for IgA. Hemolysis did not subside despite multiple transfusions and treatment lines, leading to a fatal outcome. Here, we set out to investigate underlying pathophysiological mechanisms.
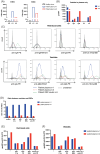
Study design and methods: To investigate the underlying pathophysiological methods, standard hematological methods were used, as well as erythrophagocytosis assays and flow cytometry using patient RBCs and plasma, to investigate anti-RBC antibodies, complement activation, and vesiculation.
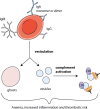
Results: Blood smear analysis revealed significant heterogeneity in RBC size and volume, and the presence of ghost cells, indicating RBC damage. While the patient's RBCs were found opsonized with IgA and IgG autoantibodies, phagocytosis by neutrophils was not induced in vitro, nor did sensitized donor RBCs with patient plasma. Using flow cytometry, we detected vesicles in the patient's plasma and observed patient plasma-induced vesiculation of healthy donor RBC. Patient plasma showed marked complement activation, and the vesicles in the patient plasma were also complement-opsonized, as well as bound by IgA, IgG, and IgM.
Conclusions: Based on these findings, we suggest vesiculation of RBCs as evidenced by the presence of vesicles and ghost cells in the patient, and subsequent complement activation induced by the vesicles. This could have driven the aggravation of the disease in this patient, resulting in a fatal outcome.
Keywords: AIHA/drug‐induced IHA; hematology—red cells; immunology (other than RBC serology).
© 2025 The Author(s). Transfusion published by Wiley Periodicals LLC on behalf of AABB.
Conflict of interest statement
Masja de Haas delivers paid consultancy for JnJ and educational services for BioRad. Josephine M. I. Vos has received the following as institutional honoraria: research support from Beigene and AbbVie/Genmab; advisory board/consultancy fees from Sanofi and Janssen; and speaker fees from BMS, Sanofi, Beigene, Novartis, and Amgen.
Figures



References
-
- Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. 2021;385(15):1407–1419. - PubMed
-
- Sokol RJ, Hewitt S, Booker DJ, Bailey A. Red cell autoantibodies, multiple immunoglobulin classes, and autoimmune hemolysis. Transfusion. 1990;30(8):714–717. - PubMed
-
- Sokol RJ, Booker DJ, Stamps R, Booth JR, Hook V. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37(2):175–181. - PubMed
-
- Bardill B, Mengis C, Tschopp M, Wuillemin WA. Severe IgA‐mediated auto‐immune haemolytic anaemia in a 48‐yr‐old woman. Eur J Haematol. 2003;70(1):60–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

