Frailty and cardiovascular safety of JAK inhibitors versus TNF inhibitors in rheumatoid arthritis: a real-world comparative study of drug effects and patient profiles
- PMID: 40351437
- PMCID: PMC12062075
- DOI: 10.3389/fphar.2025.1565909
Frailty and cardiovascular safety of JAK inhibitors versus TNF inhibitors in rheumatoid arthritis: a real-world comparative study of drug effects and patient profiles
Abstract
Objective: The aim of this study was to comparatively assess the risk of cardiovascular events (CVEs) in rheumatoid arthritis (RA) patients treated with Janus kinase inhibitors (JAKis) or tumor necrosis factor inhibitors (TNFis) and to explore the interactions with patient profiles [including age, baseline cardiovascular (CV) risk, and frailty, which is a state of decreased physiological reserve, assessed using a validated frailty index (FI) for healthcare administrative databases (AHDs)].
Methods: This retrospective study was based on AHDs of the Lombardy region, Italy (from 1st January 2020 to 31st December 2023). Cox regression models, both crude and adjusted, were applied to estimate the association between treatments and outcomes [CVEs, major adverse cardiovascular events (MACEs), and thromboembolic events (TEs)]. We tested the interaction between the drug treatment and the regulatory agency prescription rule changes [before or after 06th July 2021, the date of the first European Medicine Association (EMA) pronouncement on tofacitinib safety] or patient profiles.
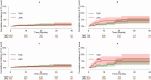
Results: We identified 7,541 therapeutic courses in 5,563 patients: 2,343 started as TNFi users, 1,443 as JAKi users, and 1,777 started with other drugs (1,459 days of follow-up). The crude incidence rates (IRs) for new CVEs were 16.6 [95% confidence intervals (95% CI): 12.8-21.2] and 18.6 (95% CI: 14.2-23.9) per 1,000 person-years (PYs) for TNFi and JAKi users, respectively. Exposure to JAKis was not associated with a significantly increased risk of CVEs [adjusted hazard ratio (HR): 0.92; 95% CI: 0.64-1.32], MACEs (adjusted HR: 0.71; 95% CI: 0.37-1.33), or TEs (adjusted HR: 1.53; 95% CI: 0.65-3.65) compared to TNFis. Each 0.1-point increment of the FI significantly increased the HR for new CVEs (HR: 1.80; 95% CI: 1.48-2.19), MACEs (HR: 1.66; 95% CI: 1.10-2.51), and TEs (HR: 1.69; 95% CI: 1.03-2.78). When assessing the interaction between the period of drug delivery and the treatment with JAKis on the risk of new CVEs, no significant interaction was observed (p = 0.838), while the interaction was statistically significant for baseline CV risk (p = 0.007).
Conclusion: RA patients treated with JAKis in real-world settings have a risk of developing CVEs no higher than those of TNFi users, but potential signals remain for TEs, even if the sample was not sufficiently powered. Patient profiles, particularly the frailty, have a more substantial impact on the risk of CVEs than the specific disease-modifying anti-rheumatic drug (DMARD) choice.
Keywords: JAK inhibitors; TNF inhibitors; cardiovascular events; frailty; healthcare administrative databases; rheumatoid arthritis.
Copyright © 2025 Silvagni, Bortoluzzi, Occhino, Bellelli, Garaffoni, Delvino, Leoni, Valsecchi, Scirè and Rebora.
Conflict of interest statement
ES has received research support from AbbVie and Lilly and consulting/speaker’s fees from AbbVie, Galapagos, Lilly, Novartis. CAS has received research support from AbbVie, Lilly, and Galapagos and consulting/speaker’s fees from AbbVie, Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Argnani L., Zanetti A., Carrara G., Silvagni E., Guerrini G., Zambon A., et al. (2021). Rheumatoid arthritis and cardiovascular risk: retrospective matched-cohort analysis based on the RECORD study of the Italian society for rheumatology. Front. Med. 8, 745601. 10.3389/fmed.2021.745601 - DOI - PMC - PubMed
-
- Aymon R., Mongin D., Bergstra S. A., Choquette D., Codreanu C., Cock D. D., et al. (2023). Evaluation of discontinuation for adverse events of JAK inhibitors and bDMARDs in an international collaboration of rheumatoid arthritis registers (the ‘JAK-pot’ study). Ann. Rheum. Dis. 83, 421–428. 10.1136/ard-2023-224670 - DOI - PMC - PubMed
-
- Bellelli G., Zucchelli A., Benussi A., Pinardi E., Caratozzolo S., Ornago A. M., et al. (2023). Assessing frailty at the centers for dementia and cognitive decline in Italy: potential implications for improving care of older people living with dementia. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 44, 3509–3514. 10.1007/s10072-023-06885-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

