Structural Heart Interventions in Patients with Left Ventricular Assist Devices
- PMID: 40351683
- PMCID: PMC12059792
- DOI: 10.31083/RCM27964
Structural Heart Interventions in Patients with Left Ventricular Assist Devices
Abstract
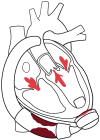
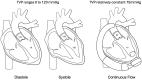
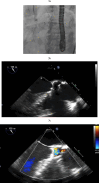
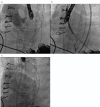
Left ventricular assist devices (LVADs) have changed the landscape for patients with advanced heart failure (HF). With advances in pump design and management, patients with LVADs are living longer with improved quality of life despite having more comorbidities and complex structural heart disease. As such, HF cardiologists and surgeons collaborate more frequently with structural heart interventionalists to address the complex problems of patients with LVADs who present at different points of failure in their circuits. Unlike heart transplants and total artificial heart recipients, the native heart and its components must function to maintain successful circulatory support from these assist devices. Multiple points of potential failure of the native heart and the LVAD circuit exist that can result in significant morbidity and mortality. These include regurgitant valve lesions, interatrial shunts, outflow cannula obstruction, and pump thrombosis. Transcatheter interventions can be applied and tailored specifically to the anatomy of the individual in these situations to improve the lives and outcomes of our LVAD patients. This review provides a comprehensive approach for diagnosing and treating structural heart disease associated with patients who have LVADs, focusing on multidisciplinary collaboration and individualized interventional strategies.
Keywords: left ventricular assist device; transcatheter interventions; valvular heart disease.
Copyright: © 2025 The Author(s). Published by IMR Press.
Conflict of interest statement
Authors declare the following potential conflicts of interest: GE. Consultant: Abbott Inc., DK Consultant for Edwards Life Sciences and J.C. Medical, Inc., SG. Proctor, consultant and institutional research grants: Edwards Life sciences, Medtronic and Abbott structural heart, PS. Proctor: Medtronic, JC. Proctor: Abbott. Speaker bureau: Medtronic.
Figures
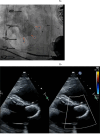

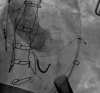



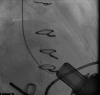

References
-
- Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno CT, et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. Journal of the American Medical Association . 2022;328:1233–1242. doi: 10.1001/jama.2022.16197. - DOI - PMC - PubMed
-
- Saeed D, Grinstein J, Kremer J, Cowger JA. Aortic insufficiency in the patient on contemporary durable left ventricular assist device support: A state-of-the-art review on preoperative and postoperative assessment and management. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation . 2024;43:1881–1893. doi: 10.1016/j.healun.2024.06.018. - DOI - PubMed
-
- Jain R, Truby LK, Topkara VK. Residual mitral regurgitation in patients with left ventricular assist device support - An INTERMACS analysis. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation . 2022;41:1638–1645. doi: 10.1016/j.healun.2022.03.002. - DOI - PubMed
-
- Veen KM, Mokhles MM, Soliman O, de By TMMH, Mohacsi P, Schoenrath F, et al. Clinical impact and “natural” course of uncorrected tricuspid regurgitation after implantation of a left ventricular assist device: an analysis of the European Registry for Patients with Mechanical Circulatory Support (EUROMACS) European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery 2021; 59: 207–216 . 2021;59:207–216. doi: 10.1093/ejcts/ezaa294. - DOI - PMC - PubMed
-
- Dimitrov K, Kaider A, Angleitner P, Schlöglhofer T, Gross C, Beitzke D, et al. Incidence, clinical relevance and therapeutic options for outflow graft stenosis in patients with left ventricular assist devices. European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery . 2022;61:716–724. doi: 10.1093/ejcts/ezab382. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

