A sensitive and selective electrochemical detection and kinetic analysis of methyl parathion using Au nanoparticle-decorated rGO/CuO ternary nanocomposite
- PMID: 40352388
- PMCID: PMC12063486
- DOI: 10.1039/d5ra00765h
A sensitive and selective electrochemical detection and kinetic analysis of methyl parathion using Au nanoparticle-decorated rGO/CuO ternary nanocomposite
Abstract
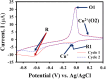
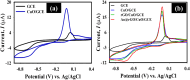
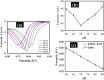
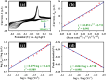
Detecting organophosphorus pesticide (OP) residues is essential for maintaining ecological integrity and monitoring public health concerns. This research developed a novel electrochemical sensor that employed composite materials based on copper oxide (CuO) nanostructures and reduced graphene oxide (rGO), customized with Au nanoparticles (AuNPs), to detect methyl parathion (MP) pesticide with high selectivity and sensitivity. The nanocomposite was synthesized in two facile steps, without the use of stabilizers or dispersants, utilizing a simple ultrasonication and photo-reduction process. Morphological analysis revealed a uniform distribution of AuNPs and rGO within the CuO nanostructure. Kinetic studies demonstrated that the electro-reduction of MP on a glassy carbon electrode (GCE) modified with Au@rGO/CuO exhibited irreversible, diffusion-controlled kinetics, with a transfer coefficient (α) value of 0.485. A sensing study employing the square wave voltammetry (SWV) technique exhibited exceptional sensitivity (3.46 μA μM-1 cm-2), with a limit of detection (LOD) of 0.045 μM. Moreover, the Au@rGO/CuO-based sensor electrode exhibited exceptional selectivity for MP in the presence of various organic and inorganic species, along with notable reproducibility, repeatability, and stability. Overall, this electrochemical method for effective MP detection suggests that the prepared nanocomposite could contribute to the development of viable electrocatalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Aloizou A. M. Siokas V. Vogiatzi C. Peristeri E. Docea A. O. Petrakis D. et al., Pesticides, cognitive functions and dementia: a review. Toxicol. Lett. 2020;326:31–51. - PubMed
-
- Sidhu G. K. Singh S. Kumar V. Dhanjal D. S. Datta S. Singh J. Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit. Rev. Environ. Sci. Technol. 2019;49(13):1135–1187.
-
- Mandal W. Fajal S. Samanta P. Dutta S. Shirolkar M. M. More Y. D. et al., Selective and sensitive recognition of specific types of toxic organic pollutants with a chemically stable highly luminescent porous organic polymer (POP) ACS Appl. Polym. Mater. 2022;4(11):8633–8644.
-
- Dutta S. Mandal W. Desai A. V. Fajal S. Dam G. K. Mukherjee S. et al., A luminescent cationic MOF and its polymer composite membrane elicit selective sensing of antibiotics and pesticides in water. Mol. Syst. Des. Eng. 2023;8(12):1483–1491.
-
- Kumaravel A. Chandrasekaran M. A novel nanosilver/nafion composite electrode for electrochemical sensing of methyl parathion and parathion. J. Electroanal. Chem. 2010;638(2):231–235.
LinkOut - more resources
Full Text Sources
Miscellaneous

