Synthesis of Δ1-Pyrrolines via Formal (3 + 2)-Cycloaddition of 2 H-Azirines with Enones Promoted by Visible Light under Continuous Flow
- PMID: 40352483
- PMCID: PMC12060062
- DOI: 10.1021/acsomega.5c01416
Synthesis of Δ1-Pyrrolines via Formal (3 + 2)-Cycloaddition of 2 H-Azirines with Enones Promoted by Visible Light under Continuous Flow
Abstract
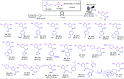
This work reports the first synthesis of Δ1-pyrrolines promoted by visible light under continuous flow, achieved through the formal (3 + 2)-cycloaddition of 2H-azirines to enones. A total of 22 examples of trisubstituted Δ1-pyrrolines were prepared in only 30 min of residence time, with 41-93% yield and diastereomeric ratios up to 7:3. Furthermore, continuous flow conditions were also effective when chalcones were used as starting materials, leading to the formation of 7 tetrasubstituted pyrroles with an overall yield ranging from 12 to 47%, via a photocatalyzed cycloaddition-oxidation sequence.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Marusich J. A.; Darna M.; Wilson A. G.; Denehy E. D.; Ebben A.; Deaciuc A. G.; Dwoskin L. P.; Bardo M. T.; Lefever T. W.; Wiley J. L.; Reissig C. J.; Jackson K. J. Tobacco’s Minor Alkaloids: Effects on Place Conditioning and Nucleus Accumbens Dopamine Release in Adult and Adolescent Rats. Eur. J. Pharmacol. 2017, 814, 196–206. 10.1016/j.ejphar.2017.08.029. - DOI - PMC - PubMed
-
- Zhou J.; Jia M.; Song M.; Huang Z.; Steiner A.; An Q.; Ma J.; Guo Z.; Zhang Q.; Sun H.; Robertson C.; Bacsa J.; Xiao J.; Li C. Chemoselective Oxyfunctionalization of Functionalized Benzylic Compounds with a Manganese Catalyst. Angew. Chem., Int. Ed. 2022, 61, e202205983 10.1002/anie.202205983. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
