Enantioselective synthesis of axially chiral P-containing allenes for allenyl chirality
- PMID: 40353065
- PMCID: PMC12064165
- DOI: 10.1016/j.checat.2024.100954
Enantioselective synthesis of axially chiral P-containing allenes for allenyl chirality
Abstract
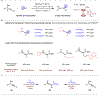
In this issue of Chem Catalysis, Wang J. and co-workers have established an efficient and practical route to construct versatile di-, tri-, and tetra-substituted phosphoryl allenes via a nickel-catalyzed propargylic substitution reaction, offering a powerful tool for the assembly of axially chiral phosphorus-containing allenes.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures
References
-
- Cabré A, Riera A, and Verdaguer X (2020). P-Stereogenic amino-phosphines as chiral ligands: From privileged intermediates to asymmetric catalysis. Acc. Chem. Res, 53, 676–689. - PubMed
-
- Li YM, Kwong FY, Yu WY, and Chan AS (2007). Recent advances in developing new axially chiral phosphine ligands for asymmetric catalysis. Coord. Chem. Rev, 251, 2119–2144.
Grants and funding
LinkOut - more resources
Full Text Sources
