Immune checkpoint inhibitors: From friend to foe
- PMID: 40353246
- PMCID: PMC12063143
- DOI: 10.1016/j.toxrep.2025.102033
Immune checkpoint inhibitors: From friend to foe
Abstract
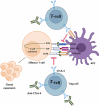
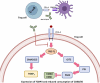
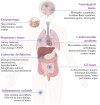
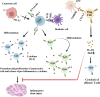
Immune checkpoints are crucial in regulating the activation of cell-mediated and humoral immune responses. However, cancer cells hijack this mechanism to evade the immune surveillance and anti-cancer response. Typically, receptors like PD-1 and CTLA4, expressed on immune cells, prevent the activation and differentiation of T cells. They also inhibit the development of autoimmune reactions. However, ligands such as PD-L1 for the receptor PD-1 are also expressed on the surface of cancer cells that help prevent the activation of anti-cancer immune responses by blocking the signalling pathways mediated by PD-1 and CTLA4. Immune checkpoint inhibitors (ICIs) have promising therapeutic efficacy for treating several cancers by activating T cells and their differentiation into effector cells against tumours. Nonetheless, hyperactivated immune cells usually contribute to detrimental issues, also known as immune-related adverse effects (IrAE). IrAEs have been observed in multiple organs, leading to neurological issues, colitis, endocrine dysfunction, renal issues, hepatitis, pneumonitis, and dermatitis. The interplay between hyperactivated T cells and Treg cells helps in orchestrating the development of autoimmunity. Moreover, the crosstalk between proinflammatory interleukins and the development of autoantibodies also mediates the multiorgan effects of ICIs in cancer patients. IrAEs are generally managed by terminating the ICI therapy, reducing the ICI dose, and by using corticosteroids to subvert inflammation. Therefore, the present review aims to delineate the impacts of ICIs on the development of autoimmune diseases and inflammatory outcomes in cancer patients. In addition, mechanistic insight involving immune cells, cytokines, and autoantibodies for ICI-mediated IrAEs will also be discussed with updated findings in this field.
Keywords: Auto-immunity; Immune checkpoint inhibitors; Immune-related adverse effects; Inflammation; Rheumatoid arthritis.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors.Front Immunol. 2022 Apr 26;13:779691. doi: 10.3389/fimmu.2022.779691. eCollection 2022. Front Immunol. 2022. PMID: 35558065 Free PMC article. Review.
-
Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events.Front Immunol. 2022 Mar 30;13:804597. doi: 10.3389/fimmu.2022.804597. eCollection 2022. Front Immunol. 2022. PMID: 35432346 Free PMC article. Review.
-
Patterns and outcomes of immune-related adverse events in solid tumor patients treated with immune checkpoint inhibitors in Thailand: a multicenter analysis.BMC Cancer. 2021 Nov 25;21(1):1275. doi: 10.1186/s12885-021-09003-z. BMC Cancer. 2021. PMID: 34823493 Free PMC article.
-
Immune-related adverse events in various organs caused by immune checkpoint inhibitors.Allergol Int. 2022 Apr;71(2):169-178. doi: 10.1016/j.alit.2022.01.001. Epub 2022 Jan 29. Allergol Int. 2022. PMID: 35101349 Review.
-
Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer.JAMA Oncol. 2020 Dec 1;6(12):1952-1956. doi: 10.1001/jamaoncol.2020.5012. JAMA Oncol. 2020. PMID: 33119034 Free PMC article.
References
-
- Weber J.S., Hodi F.S., Wolchok J.D., Topalian S.L., Schadendorf D., Larkin J., Sznol M., Long G.V., Li H., Waxman I.M., Jiang J., Robert C. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. JCO. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. - DOI - PubMed
-
- Tarhini A.A., Lee S.J., Hodi F.S., Rao U.N.M., Cohen G.I., Hamid O., Hutchins L.F., Sosman J.A., Kluger H.M., Eroglu Z., Koon H.B., Lawrence D.P., Kendra K.L., Minor D.R., Lee C.B., Albertini M.R., Flaherty L.E., Petrella T.M., Streicher H., Sondak V.K., Kirkwood J.M. Phase III study of adjuvant Ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American Intergroup E1609. JCO. 2020;38:567–575. doi: 10.1200/JCO.19.01381. - DOI - PMC - PubMed
-
- Weber J., Mandala M., Del Vecchio M., Gogas H.J., Arance A.M., Cowey C.L., Dalle S., Schenker M., Chiarion-Sileni V., Marquez-Rodas I., Grob J.-J., Butler M.O., Middleton M.R., Maio M., Atkinson V., Queirolo P., Gonzalez R., Kudchadkar R.R., Smylie M., Meyer N., Mortier L., Atkins M.B., Long G.V., Bhatia S., Lebbé C., Rutkowski P., Yokota K., Yamazaki N., Kim T.M., De Pril V., Sabater J., Qureshi A., Larkin J., Ascierto P.A. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

