Association between NT-proBNP changes and clinical outcomes in paediatric patients with heart failure: Insights from PANORAMA-HF and PARADIGM-HF
- PMID: 40353367
- PMCID: PMC12287839
- DOI: 10.1002/ehf2.15326
Association between NT-proBNP changes and clinical outcomes in paediatric patients with heart failure: Insights from PANORAMA-HF and PARADIGM-HF
Abstract
Aims: The PANORAMA-HF trial demonstrated significant N-terminal pro-B-type natriuretic peptide (NT-proBNP) reductions in paediatric patients with left ventricular systolic dysfunction with sacubitril/valsartan or enalapril treatment over 52 weeks. This post hoc analysis aims to correlate changes in NT-proBNP levels with clinical outcomes in PANORAMA-HF patients receiving either sacubitril/valsartan or enalapril. Additionally, NT-proBNP reductions in the paediatric population were compared with a subset of adult heart failure with reduced ejection fraction (HFrEF) patients from the PARADIGM-HF trial.
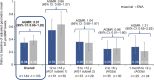
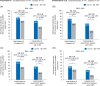
Methods and results: This post hoc analysis utilized data from Part 2 of the PANORAMA-HF trial. Associations between baseline NT-proBNP levels, changes post-baseline and the risk of HF clinical events in paediatric patients on sacubitril/valsartan or enalapril were assessed. The paediatric HF population from PANORAMA-HF was categorized into age groups (AG): AG1 (aged 6 to <18 years), AG2a (aged 2 to <6 years) and AG3a (aged 1 month to <2 years). The Cox proportional hazard model evaluated the relationship between NT-proBNP and clinical outcomes. Analysis of 361 paediatric patients (sacubitril/valsartan, n = 179; enalapril, n = 182) demonstrated overall higher baseline NT-proBNP levels in younger AGs. At Week 52, both treatment groups exhibited reduced NT-proBNP levels across all AGs. Reductions were comparable between sacubitril/valsartan and enalapril, with a numerically greater reduction observed in adult patients versus children. Strong associations between NT-proBNP levels and HF clinical outcomes were observed in paediatric populations in PANORAMA-HF and in adult DCM patients with HFrEF in PARADIGM-HF. Doubling of NT-proBNP levels was associated with a ≥1.7-fold increased risk of HF clinical events, while halving of the levels correlated with a 52% reduction in the risk of clinical events.
Conclusions: This is the first prospective, randomized large-scale study to demonstrate a strong correlation between NT-proBNP levels and risks of HF clinical events in paediatric patients with HF.
Keywords: Left ventricular systolic dysfunction; NT‐proBNP; Paediatric heart failure; Sacubitril/valsartan.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
RS: Consultant for Novartis, American Regent Inc., CRI Biotech, and Rocket Pharmaceuticals. JG: Employee of Novartis Pharmaceuticals, East Hanover, New Jersey, USA. TG: Employee of Novartis Pharma AG, Basel, Switzerland and owns its shares. SSY: Employee of Novartis Pharmaceuticals Corporation, US, and owns stocks in Novartis Pharma AG, Basel, Switzerland. SZ: Employee of Novartis, Shanghai, China. MFP: Employee of Novartis Pharmaceuticals, East Hanover, New Jersey, USA. DB: Reports consulting fees from Novartis. PFK: Reports consulting fees from Novartis, Rocket Pharmaceuticals. MB: Member of Data Safety Monitoring Board and Advisory Board for this study. CM: Nothing to disclose. AC: Nothing to disclose. CC: Data and Safety Monitoring Board chairman at the Mayo Research Foundation and has participated in an advisory board for CareDx. YL: Nothing to disclose. GG: Nothing to disclose. JKW: Nothing to disclose. AJ: Nothing to disclose. JR: Reports receiving consulting fees from Bayer, Enzyvant, Merck, AskBio, American Regent, Bristol Myers Squibb.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
