IFNβ absence compensates for LAT functions in latency reactivation and T cell exhaustion
- PMID: 40353667
- PMCID: PMC12172465
- DOI: 10.1128/jvi.00374-25
IFNβ absence compensates for LAT functions in latency reactivation and T cell exhaustion
Abstract
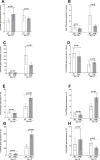
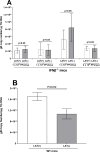
Type I interferons (IFNs) generate strong antiviral immunity following viral infection in mice and humans. These type I IFNs are encoded by at least 14 IFNα genes and a single IFNβ gene. We showed that the absence of one of the IFNα genes (IFNα2A-/-) affected latency levels in herpes simplex virus type 1 (HSV-1) ocularly infected mice but not in infected control mice, and the absence of IFNα2A did not affect viral reactivation in ocularly infected mice. Since the role of IFNβ in HSV-1 latency reactivation and the potential effect of latency-associated transcript (LAT) on IFNβ activity is not known, we ocularly infected IFNβ-/- mice with different doses of LAT-plus [LAT(+)] and LAT-minus [LAT(-)] viruses. Wild-type (WT) control mice and IFNβ-/- mice were infected similarly. Virus titers in the eye, viral and cellular transcripts in the eye and trigeminal ganglia (TG) of infected mice on days 3 and 5 post-infection, eye disease, survival, latency-reactivation levels, and T cell exhaustion were measured in latently infected mice. Virus replication and viral and cellular transcripts in the eye of infected IFNβ-/- mice were similar to those in WT mice, while eye disease and survival in IFNβ-/- mice differed significantly from WT mice. WT mice infected with LAT(-) virus showed reduced latency, slower reactivation, and less T cell exhaustion than mice infected with LAT(+) virus. However, using different doses of each virus, latency levels, time of reactivation, and T cell exhaustion were similar between LAT(+) and LAT(-) viruses. These results suggest that the absence of IFNβ expression compensates for the function of LAT with regard to levels of latency, T cell exhaustion, and reactivation but does not affect viral and cellular transcripts during primary infection.IMPORTANCEInterferon β (IFNβ) is a type I interferon that plays an important role in controlling primary herpes simplex virus type 1 (HSV-1) infection. To evaluate the importance of IFNβ on HSV-1 latency reactivation and its relationship to LAT, we infected IFNβ-/- mice with LAT(+) and LAT(-) viruses. In the absence of IFNβ, latency levels in mice infected with LAT(-) virus were similar to those of mice infected with LAT(+) virus. The absence of IFNβ also reduced the time of reactivation in mice infected with LAT(-) virus to that of LAT(+) virus. Our results show a strong correlation between the functions of LAT and IFNβ during latent but not primary stages of HSV-1 infection.
Keywords: CD8; IFNβ; PD-1; exhaustion; eye disease; knockout; latency; ocular infection; reactivation; type 1 interferon; virus replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Jamali A, Hu K, Sendra VG, Blanco T, Lopez MJ, Ortiz G, Qazi Y, Zheng L, Turhan A, Harris DL, Hamrah P. 2020. Characterization of resident corneal plasmacytoid dendritic cells and their pivotal role in herpes simplex keratitis. Cell Rep 32:108099. doi: 10.1016/j.celrep.2020.108099 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

