Repurposing Amiodarone for Bladder Cancer Treatment
- PMID: 40353763
- PMCID: PMC12134865
- DOI: 10.1158/2767-9764.CRC-24-0433
Repurposing Amiodarone for Bladder Cancer Treatment
Abstract
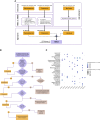
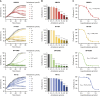
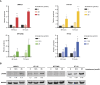
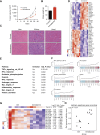
Cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy is the main treatment for muscle-invasive bladder cancer (MIBC). However, low survival rates highlight the necessity for new therapeutic strategies. Drug repurposing has emerged as a promising approach in cancer treatment, with various studies proposing the use of existing drugs for the treatment of bladder cancer. In this context, we previously established an in silico repurposing strategy using patient omics signatures, identifying drugs and compounds with the potential to reverse nonmuscle-invasive bladder cancer (NMIBC) to less aggressive subtypes. In the present study, we expanded our in silico approach to verify a list of compounds with potential antitumor activity against MIBC. We investigated the efficacy of the predicted candidates in a group of different bladder cancer cell lines, including NMIBC and MIBC. The most potent compound for decreasing cell viability was amiodarone, an antiarrhythmic drug widely used in the field of cardiology. Amiodarone reduced cell proliferation and colony formation capacity, with a stronger effect on the most aggressive invasive models, validating our repurposing pipeline. The drug additionally induced cell death and inhibited the activity of mTOR and its target protein S6, suggesting that the anticancer effect of the drug is, in part, mediated by inhibition of the mTOR signaling pathway. Furthermore, the administration of amiodarone in a xenograft MIBC mouse model reduced tumor growth without inducing toxicity. Altogether, we demonstrated that amiodarone is a potential repurposed drug for bladder cancer, which might be especially effective in MIBC.
Significance: Treatment of advanced bladder cancer remains a therapeutic challenge in urological oncology. In order to make more drugs available to patients in the future, we identified amiodarone, a repurposed drug used in cardiology as a compound that inhibits bladder cancer in vitro and in vivo.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
F.J. Roa reports grants from Eurostars—BMBF (Germany, 01QE2010A) and FFG during the conduct of the study. F. Handle reports personal fees from the Medical University of Innsbruck during the conduct of the study and that he is the owner and CEO of XPseq Analytics GmbH. M. Mokou reports grants from Mosaiques diagnostics during the conduct of the study as well as other support from Mosaiques diagnostics outside the submitted work and has a patent to EP4428869A1 pending. M. Frantzi reports personal fees from Mosaiques diagnostics GmbH during the conduct of the study. A. Latosinska reports personal fees from Mosaiques diagnostics GmbH and grants from BMBF (01QE2010A)/Eurostars (E! 113726) during the conduct of the study. H. Mischak reports grants from BMBF during the conduct of the study; has a patent to PCT/EP2024/055936 pending; and that he is the co-founder and co-owner of Mosaiques diagnostics. No disclosures were reported by the other authors.
Figures


References
-
- American Cancer Society . Cancer Facts & Figures 2024. Atlanta: American Cancer Society; 2024.
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. . Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74:229–63. - PubMed
-
- Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME, et al. . Bladder cancer. Nat Rev Dis Primers 2017;3:17022. - PubMed
-
- Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. . Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:244–58. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

